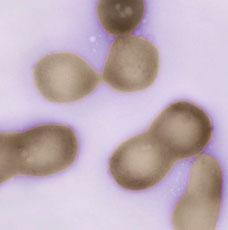
Venter Creates First Synthetic Self-Replicating Bacteria from Scratch

Share
*Update: We've included the Science interview with Venter after the break.
Craig Venter wants to program life the way we program computers, and today he announced a momentous win: the first synthetic self-replicating bacterium. The J. Craig Venter Institute (JCVI) used the four types of chemicals that make up DNA, and complex assembly methods utilizing yeast cells, to 'program' the 1.08 million base pairs that make up the genome for the bacteria cell. As described in the journal Science, the result was a synthetic copy of the Mycoplasma mycoides, dubbed M. mycoides JCVI-syn1.0, that can grow and divide like normal. The little "1.0" highlights the vast potential of Venter's project, as JCVI will be able to update and improve their synthetic organism base pair by base pair, gene by gene. Computers can now program sustainable synthetic life - welcome to the future.
JCVI, funded through Synthetic Genomic Inc, has been working on the concept of programming life for more than a decade. In 2003 they had programmed the first synthetic virus. In 2008 they created a synthetic bacteria genome but were unable to get it to thrive inside a cell. In Venter's most recent presentation at TED, he described these efforts and said it was possible that 2010 would see the arrival of the first fully synthesized organism. Clearly he has delivered on that prediction with flying colors. The M. mycoides JCVI-syn1.0 represents a whole new level of synthetic biology. Science interviewed Venter about the endeavor via Skype:
The main methodology for synthetic biologists up to this point has been 'cut and paste'. The DNA of one organism has been carefully selected and transplanted into another. In this way, scientists have been able to give the properties of one organism to another. This 'gene-splicing' or 'bio-hacking' is far from crude, and represents a growing and important field of study. MIT has an expanding databank of DNA, its Registy of Standard Biological Parts, that can be used to transform organisms (almost always bacteria) for synthetic biology projects. This methodology is at the heart of iGEM, OpenWetWare, and virtually every single other synthetic bio endeavor we've discussed at Singularity Hub.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Venter just made that entire field of study look like finger painting next to a Picasso. JCVI's approach to synthetic biology isn't hacking, it's programming from the ground up. Yes, this first bacterium was just a copy of a natural organism. But that copy was assembled base pair by base pair. In the future, instead of pain-stakingly slicing in genes from other bacteria, Venter can just change a few parameters in his computer software. When fully developed this technology will let you 'code' a new organism. Can you imagine the power of that kind of control? Forget finger painting and Picasso, this is the difference between playing Frankenstein and playing God.
For those who missed Venter's TED talk on the subject, let me describe briefly what this amazing process entails. The underlying concept is that the software of life (DNA) will build its own hardware (the cell). Using the four basic chemicals of DNA (the G,A,C,T) small snippets of genetic code are implanted into yeast cells. The yeast cells act as little factories, assembling these snippets into overlapping segments of DNA. When the newly programmed bacteria genome is assembled, it can be transplanted into a host bacterium where it takes over, rewriting the cell to create the new synthetic organism. Thus, while this is called a synthetic form of life, many natural forms of life are necessary to assemble it and provide its cytoplasm body. Keep in mind though that when the M. mycoides JCVI syn1.0 forms a colony, not a single bacterium will contain the proteins of the assembling yeast or host bacterium. The programmed DNA is king.
Despite JCVI's amazing innovations with their new approach to synthetic biology, it's likely that 'bio-hacking' will remain dominant in the near term. There are many more teams working with that technique, and years of experience with getting it to work. Even after the JCVI approach picks up speed, it is likely to copy many of its ideas from the greatest DNA programmer humans have ever seen: nature. In other words, most of the first changes to the 1.0 will probably be adoptions of known DNA segments from other natural organisms, so this will not seem so different from the bio-hacking approach. Eventually however, programming DNA is going to lead to an explosion of new organisms. Rather than slicing in a few genes at a time, JCVI and others will be able to 'write' genetic code. With enough computing power they would also be able to simulate these changes before 'booting' them up into real cells, but I'm not sure which approach (simulate or guess and check) will end up being more cost effective. Either way, we'll be building new life from the ground up, custom building organisms for our needs.
Biofuels, vaccines, antibiotics, synthetic insulin, advanced solar cells...there's an almost limitless amount of possible 'first applications' for this new technology. JCVI is already working with Exxon to create algae fuels though maybe they'lll take a stab at all of these ideas. It could take some time. As always with biotechnology, and with synthetic biology in particular, there are many safety and administrative hurdles to clear before lab work can be converted into marketable products. Will these synthetic species cause ecological damage if released out of the lab? Can we build fail-safe DNA (a genetic kill-switch) into these organisms to prevent their accidental or intentional misuse? Will customers trust a non-natural organism for biofuels/vaccines/whatever? Each of these questions will need to be addressed before we can reap the benefits of Venter's work. One day soon, though, humanity will take its first cautious steps through the door of fully synthetic biology. When that happens we will see amazing and powerful changes. This could be the defining technology of the 21st century. May 20th, 2010. Remember the date.
[image credits: J. Craig Venter Institute]
[video credit: Science]
[source: JCVI, JCVI press release, Science]
Related Articles

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Google DeepMind AI Decodes the Genome a Million ‘Letters’ at a Time
Scientists Turn Mysterious Cell ‘Vaults’ Into a Diary of Genetic Activity Through Time
What we’re reading