Crizotinib Targets Gene To Stop Lung Cancer Tumors in 90% of Treated Patients

Share
The drug Crizotinib (PF0234-1066) was shown to shrink or stabilize tumors in about 90% of the patients who took it. Dr. Yue-Jue Bang of Seoul National University presented these results at the annual meeting of the American Society of Clinical Oncology in Chicago last week. Crizotinib works by inhibiting a genetic mutation, EML4-ALK, that is found in lung cancer cells. Roughly 4% of all lung cancer patients have this gene in their tumors and would be likely to benefit from Crizotinib. Pfizer sponsored the research, is proceeding with phase III trials, and is likely to seek FDA approval as early as next year (Reuters). As the EML4-ALK gene was only recently identified in 2007, the work with Crizotinib shows how quickly genetic technologies may be parlayed into powerful and accessible medical therapies.
Lung cancer is one of the biggest killers in the industrialized world. According to the National Cancer Institute, almost 220,000 new cases of lung cancer occur every year in the United States alone. Of these, nearly 10,000 would have the EML4-ALK gene, making them ready targets for the Crizotinib drug. That may seem like a small fraction...and it is. Luckily, Crizotinib isn't alone. Tarceva (from Genentech) and Iressa (from Astra Zeneca) work in a similar fashion by targeting different mutated genes in tumors. With each new gene identified we can presumably develop another drug to target it, tightening the net until the majority of lung cancers have highly effective therapies. This is a sign that large genetic studies are going to pay big dividends in the years ahead.
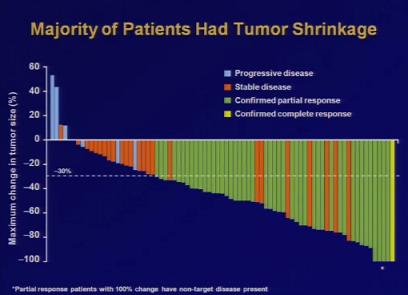
Dr. Bang's Crizotinib trials focused on 82 patients with advanced lung cancer, the majority of whom had already tried a cancer treatment (i.e. chemotherapy) with minimal positive results. As such, Bang expected only about 10% to respond well to Crizotinib. 90%, however, saw some benefits from taking Crizotinib orally, 57% saw significant improvements, and a few had their tumors disappear altogether. You can see Dr. Bang's presentation at the ASCO press conference in a video on their site (direct link here).
Given Crizotinib's promising results, it's no surprise that Pfizer is working to get it to market as soon as possible. They are working with Abbott Molecular to develop a test to quickly identify which patients have the EML4-ALK gene in their tumors. Meanwhile, phase III clinical trials for Crizotinib are already in the works, as are safety tests with larger sample sets. Currently observed side effects include nausea (more than half of patients), diarrhea, and vomitting. According to the ASCO, however, those patients in the trial who had already undergone chemotherapy preferred these effects to ones generated by their chemotherapy drugs. Clearly we still need to verify that Crizotinib is better than alternative treatments available today, but these early results are a very positive sign of the drug's potential.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Singularity Hub really isn't in the business of commenting on every cancer drug that gets released, but Crizotinib is something special. As we already mentioned, it's one of a series of new drugs that will target specific genetic mutations in cancers. Just as importantly, Crizotinib demonstrates the remarkable speed in which a newly discovered genetic markers for cancer can be translated into a powerful therapy. The work identifying EML4-ALK was published in 2007, and Pfizer might be pursuing FDA approval in early 2011. That's less than a five year turn around! Not all drugs will have such a fast-track to consumer availability, but it gives me hope that many of the breakthrough discoveries I see everyday will be arriving in the doctor's office very soon.
[image credits: Wiki Commons, Yue-Jue Bang]
Source: reuters, asco, bang et al, asco 2010, pfizer
Related Articles
Single Injection Transforms the Immune System Into a Cancer-Killing Machine
This Light-Powered AI Chip Is 100x Faster Than a Top Nvidia GPU
This Week’s Awesome Tech Stories From Around the Web (Through December 20)
What we’re reading