Pharm. Company Roche Blocking Blindness Cure. Why?

Share
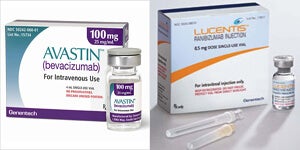
Many great scientific discoveries were born out of pure accident. How about curing blindness in only one or two treatments with a drug that was originally designed to combat cancer? What if it only cost around the same as taking your family to the movies? Impressed yet? You should be. Doctors have been using Avastin (bevacizumab), an anti-cancer drug, to treat certain types of blindness, such as vascular retinopathy, and the initiative paid off more than anybody ever imagined, the drug being 20% more effective than conventional laser therapy. However, there are always obstacles to great ideas, and this time they are human rather than technical. Roche Group, the company behind Avastin, simply does not support its use for treatment of retinopathy. Why? Roche’s official position is that they are concerned about patient safety since Avastin was not designed to be used for eye conditions. However, perhaps it has less to do with supposed safety concerns and more with the fact that Avastin costs approximately forty times less than Lucentis (ranibizumab), Roche’s officially supported retinopathy treatment?
Avastin was originally developed as a treatment for colon cancer. However, unlike the traditional chemotherapy, it works by preventing growth of capillaries in cancer tissue. Since cells receive the necessary nutrients through the blood, halting proliferation of blood vessels effectively starves the cancer until it dies off naturally. The idea to use Avastin to treat vascular retinopathy, a type of blindness caused by overcrowding of blood vessels in the retina, seems only natural as the next logical step, as many doctors have figured out, successfully treating age-related macular retinopathy in pre-maturely born babies. By using Avastin to stop the unchecked growth of blood vessels, doctors were able to prevent the degeneration of the retina, sometimes in only one treatment.
However, since Roche does not seem to be too keen on approving Avastin’s use on the eyes, doctors undertake such treatment at their own risk. The treatment of vascular retinopathy with Avastin remains off-label and unofficial. Of course, all is not lost: Roche is coming to the rescue with Lucentis, a patented retinopathy treatment from the makers of Avastin. The only problem is that Lucentis is nothing more than a smaller derivative of Avastin’s active compound. Sure, it has been subjected to a technique called affinity maturation, which, theoretically, makes it bind more strongly to the blood vessel proteins, but on a practical level, it does not seem to be all that more effective. In fact, the only practical difference between Avastin and Lucentis, when it comes to the treatment of vascular retinopathy, is the price. Avastin costs an average $40 per dose as opposed to Lucentis’ $1600.
Doctors both in the United States and Britain have been trying for many years to get Roche to organize clinical trials to compare the efficacy of Avastin and Lucentis, but Roche has been reluctant for obvious reasons. A recent study conducted by the National Eye Institute (NEI), which included over fifty-five participant medical centers, is supposed to finally put an end to the controversy of which drug to use to treat eye conditions. The study was only finished in February with the results showing no difference in efficacy between Avastin and Lucentis in treating vascular retinopathy. A smaller scale study was done in parallel in Boston University School of Medicine that reached the same conclusion.
All of this really begs the question of just how genuine the pharmaceutical industry is. It is no secret that pharmaceutics is an expensive industry, especially in America. The United States spends the most on healthcare out of the developed countries, in large part because of medication, and still manages to have the highest rates for infant mortality and diabetes. Incidentally, the United States is also the only country in the world that allows advertising of medicines on public television. It is this practice that inflates the price of drugs for the average consumers – the marketing budget for a particular solution is usually always factored into the manufacturer’s price. This and other business practices hike the prices of medicines to obscene amounts even though the actual drug costs cents per pill to produce.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


However, much more is at stake here than pharmaceutical companies trying to recuperate the indirect costs of production by charging a higher price for the drug. The most insane fact in this whole controversy of Avastin vs. Lucentis was that Roche blatantly refused to approve, or even test, a product that was obviously effective, all the while trying to push through an almost exact copy for forty times the cost. Of course, in a capitalist economy, everyone is within their right to make a profit on a product of their making, but therein also lies the problem. The chief priority of any private company is not to actually produce anything, but to make a profit on whatever it is producing.
Of course, the study that was conducted by NEI should clear everything up in this case, but who can really be sure that other companies and other medications are not involved in similar incidents? How can the person paying for a drug know that he is really paying what the drug is actually worth? Perhaps this is a good moment to take a closer look at the pharmaceutical industry and possibly reform or tighten some of their regulations. Hopefully, this case will be a strong stimulus to launch such a reform and see that it is carried out to completion. Sadly, though, few of us believe that will actually happen.
[Source: The Guardian, National Eye Institute, Roche Group, Visual Economics]
[Image Credit: Cary Silverman]
I enjoy all types of futurology. I especially enjoy staying up to date with the latest advancements in machine learning and artificial intelligence. You can usually find me roaming the depths of the internet.
Related Articles

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Scientists Send Secure Quantum Keys Over 62 Miles of Fiber—Without Trusted Devices
This Week’s Awesome Tech Stories From Around the Web (Through February 7)
What we’re reading