
Each year in the United States about 325,000 people die due to sudden cardiac arrest (SCA). The vast majority of arrests occur at home, far from medical help which, if not delivered within minutes, normally results in death. Now an implantable defibrillator has just received FDA approval and could be the difference between life and death for many.
Boston Scientific Corporation won a major victory Sept. 28 with FDA approval of its subcutaneous implantable defibrillator, S-ICD. The company already supplies patients with ICDs, another kind of implantable defibrillator that connects to the heart through a series of insulated wires. What makes the new S-ICD defibrillator even better is that there are no wires needed – it is able to deliver electrical shocks to the heart without them. The new system not only simplifies implantation (attaching the ICD’s wires requires an x-ray procedure to visualize the beating heart), it also provides a safer alternative to many patients for which ICD is deemed too invasive.
When the heart stops beating during sudden cardiac arrest (SCA), death will likely result if treatment doesn’t occur within 10 minutes. The arrests are brought on by problems with the heart’s electrical activity that result in abnormal beating patterns. These abnormal beating patterns, or arrhythmias, can be too fast, too slow, or irregular, but all carry with them the risk for sudden cardiac arrest. The important role for electrical activity differentiates an SCA from a heart attack, which occurs when blood flow to the heart is blocked. By delivering an electric pulse to the heart, defibrillators reset the heart’s electrical activity and allow it to beat normally again. The major advantage of implantable defibrillators is their ability to monitor the electrical activity of the heart and deliver corrective pulses when needed, all without the help of medical professionals.
The recently approved device has two major components. A pulse generator, implanted at the side of the chest, provides the system with power, monitors heart activity, and delivers electric shocks if abnormal activity is detected. And an electrode, placed next to the breastbone, enables the pulse generator to both sense cardiac rhythm and deliver the shocks.
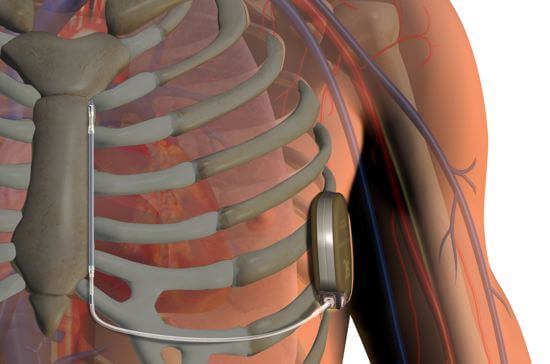
FDA approval comes on the heals of a clinical trial completed this May that showed the S-ICD system was both safe and effective for patients at risk for sudden cardiac arrest. The trial included 321 patients implanted with the device who were observed for 180 days. It was also shown to be easily implantable, needing only anatomical landmarks and no medical imaging.
Spotting the opportunity, Boston Scientific gained the defibrillator earlier this year when they acquired San Clemente, California-based Cameron Health, Inc., one month after the successful trial.
The approval means Americans can now enjoy a system that had previously been approved in many European countries and in New Zealand. To date, over 1,400 patients around the world have already received the device.
With its capacity to both monitor the heart and shock it back to health, the S-ICD is one of just a few medical devices that both watches over and care for us – autonomously. Another, the Silicon Pancreas is an implantable chip that monitors the blood glucose levels of diabetics and releases appropriate amounts of insulin or glucagon, normally produced by pancreatic cells in healthy individuals, to keep blood glucose under control.
Of course, other medical technologies have made it unnecessary to have a heart at all. But as more implants like the S-ICD defibrillator and the Silicon Pancreas are developed we move closer to the day when virtually all of our vital functions are, not only continuously monitored, but readjusted when they begin to falter.
[image credits: Boston Scientific]
images: Boston Scientific


