In the wildly popular HBO show “True Blood”, the creation of synthetic blood allows vampires to join the human society as they no longer need to feed on real, human blood. And while vampires are still only found in the fictional world, artificial blood is making its way into the real world. Known somewhat morbidly as “blood pharming”, this idea looks promising as a viable alternative to producing human blood without the human! The technology is premised on taking hematopoietic stem cells and coaxing them to become red blood cells that can be transfused during treatment of traumatic injuries or during surgeries where blood loss is unavoidable.
The Defense Advanced Research Projects Agency (DARPA), the arm of the Pentagon responsible for research, was originally interested in pursuing this technology as a way to ensure that injured soldiers had access to fresh blood, somewhat of a rare commodity on the battlefield. According to the National Trauma Institute, over 25% of military casualties required a blood transfusion upon reaching a medical facility. In the civilian arena, “after a traumatic injury, hemorrhage [profuse loss of blood from a ruptured blood vessel] is responsible for over 35% of pre-hospital deaths and over 40% of deaths within the first 24 hours, second only to the rates of death due to severe central nervous system injury.” And while these exact figures may be news to you, the potential for blood loss to lead to a poor prognosis is surely not.
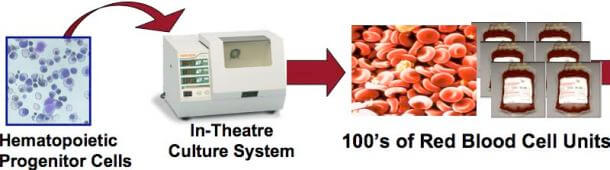
Ok, so what’s the good news? Two years ago, DARPA awarded a $1.95 million grant to a Cleveland company called Arteriocyte, which was developing technology that could quickly transform stem cells into red blood cells. The idea was to develop a system that could produce an almost limitless amount of universal donor blood (O-negative) in remote areas. And earlier this month, Arteriocyte submitted samples of their artificially produced blood to the Food and Drug Administration (FDA), seeking approval before making the technology available to both the civilian and military sectors. While we have reported attempts by other companies to produce artificial blood, Arteriocyte is clearly ahead of the pack.

Initially created at Johns Hopkins University, Arteriocyte’s technology is called NANEX and it essentially functions as the bone marrow, where red blood cells originate. Hematopoietic cells isolated from umbilical cord blood are cultured in an environment that provides them with all the nutrients and molecular signals they need to develop into red blood cells. Currently, it takes about 3 days to generate up to 20 units of transfusion-ready blood from a single unit of umbilical cord blood. For reference, it’s estimated that on average, a soldier injured in combat requires 6 units of blood during treatment. For now, the company’s claims that their pharmed blood is functionally indistinguishable from what is currently coursing through your veins remains to be validated, but the science behind the technology looks solid. Further, the current cost of pharmed blood makes it impractical for widespread use. Arteriocyte says that it costs approximately $5000 per unit; however, increasing the scale of production should bring the price per unit down. The only thing needed to scale up production is approval by the FDA, and experts currently estimate that it may take 3 – 5 years for the approval process to be completed.
If blood pharming is proven to be a legitimate way to produce human blood, then we may be witnessing a medical revolution. As you can imagine, there would be scores of advantages to using this system over the current system that depends on human donors. First, you would completely eliminate the chances of transmitting blood-borne diseases from a donor to a recipient. While rigorous testing of the blood supply has already achieved very low levels of contaminants (at least in the Western world), it is not 100% safe. Further, producing blood on demand could eliminate waste that is inevitable with our current system. By the Red Cross’s standards, the shelf-life for a unit of human-donated blood is 42 days, though some research suggests that any blood older than 28 days may carry increased risk of complications if transfused. And finally, there would be huge savings monetarily as you would eliminate the need to collect, test, store, and transport blood derived from human donors.
In an ideal world, “traffic accidents” and “war” would be terms relegated to the distant past, something we look back on and wonder about in bafflement. Unfortunately, these tragedies will likely continue to be part of the human experience for some time. And while the technology being developed by Arteriocyte won’t solve these grand challenges, it will reduce the number of lives lost until we find ways to purge our species of our imperfect and often dangerous tendencies.
[Sources: DARPA, Wired, Arteriocyte]
[Image credit: cnet news]


