No More Pills? Tiny Nerve-Zapping Implants to Fight Disease

Share
Imagine a future where we can treat diabetes or autoimmune disorders with an electrical zap delivered by a device no larger than a speck of dust.
The device, implanted through microsurgery, sits silently on a single nerve bundle, monitoring electrical signals sent out by the brain to itself and various organs in the body.
When it detects a problem — a rogue misfire, or a shift in activity patterns — the device powers up, sending out counter-pulses to correct the signal. In this way, it keeps your body running smoothly and disease at bay. No pills. No injections. No pain.
According to Alphabet and pharmaceutical giant GlaxoSmithKline (GSK), that future is just seven years away.
This week, Verily, Alphabet’s life science unit (formerly Google Life Sciences), teamed up with Britain’s biggest drug maker to announce their new $715 million venture Galvani Bioelectronics. With research centers based in GSK’s biotech hub in the UK and around the Bay Area, the company hopes to develop miniaturized, implantable electronic systems — dubbed “electroceuticals” — to correct irregular nerve pulses that contribute to a multitude of chronic diseases.
Hopes are high. According to Reuters, Kristoffer Famm, head of bioelectronics research at GSK and president of Galvani, says that their first products might be submitted for marketing approval as early as 2023.
Electricity as drugs
Eletroceuticals may sound futuristic, but using electricity to treat disease is nothing new — think pacemakers for correcting wonky heartbeats, or deep brain stimulation for rewiring broken neural circuits in depression and Parkinson’s disease.
It’s easy to see why electroceuticals are sparking interest. Unlike run-of-the-mill chemical drugs that act on a protein or other molecule, electrical pulses directly hack into the main language of our nervous system to change its operating instructions.
That’s a big deal. “The nervous system is crisscrossing our viscera to control many aspects of our organ function,” explains Famm in an earlier interview with Nature. Rather than using drugs, which are rarely specific for a single biological process, we could zap a major nerve and, with surgical precision, change the instructions that an organ receives and thereby alter its function.
Electricity can not only jump start a heart or jolt a brain into health, but under the right conditions, a well-placed zap may also coax resistant pancreatic cells to release insulin, persuade clenched arteries to relax, or berate hyperactive immune cells to stop attacking your own tissue.
Rather than developing a library of chemical drugs that targets individual diseases, a single electroceutical prototype could, in theory, be programmed to treat multiple diseases. According to a spokesperson from GSK, Galvani plans to tackle “a wide range of chronic diseases that are inflammatory, metabolic and endocrine disorders, which includes Type 2 diabetes,” but adding that the company hasn’t yet developed specific product plans.
Galvanizing the field
Last year already saw big wins for electroceuticals. In May, the US Food and Drug Administration approved a device that contracts airway muscles to help people with severe sleep apnea breathe properly without using an oxygen mask. A month later, the agency also gave its nod to an implantable weight-loss device that stimulates a nerve near the stomach to make a person feel full.
To really tap into the potential of electroceuticals, however, the devices will have to get much, much smaller. Nerves are incredibly compact, and unrelated circuits often run in close proximity. Because of this, electrical devices that zap a whole chunk of tissue run the risk of significant side effects — it’s like jump starting your car, but also blowing out the fuses in your entire house.
This is where Galvani comes in. By combining engineering, bioinformatics and neuroscience, the company hopes to shrink implanted devices to the size of a grain of rice. Although specific plans are still under wraps, back in 2013 GSK published a roadmap that will likely guide the fledgling company.
“Many of the stepping stones are already in place,” wrote Famm and his colleagues.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


First, the scientists will need to trace neural circuits that control disease to identify easy access points for intervention. They’ll also need to understand the signals running through those circuits in order to build a ‘dictionary’ of patterns that represent healthy and diseased states. By decoding the neural language, researchers can then program future electroceuticals to understand nerve impulses and, in turn, generate corrective pulses of their own.
Then there’s the engineering side of things. Bioengineers will need to design wireless, biocompatible microchips that can reliably perform real-time computation with low power. When implanted through keyhole surgeries, the hope is that these electroceuticals will last at least decades.
According to Famm, the first generation of marketable implants will be roughly the size of an average pill. However, eventually they’ll be smaller than a grain of rice.
That goal may not be far off, and Galvani’s got serious competition.
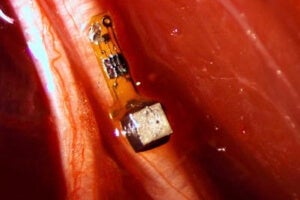
An implantable, wireless sensor like this could allow real-time monitoring of nerve or muscle activity anywhere in the body, using external ultrasound to power and read out voltages. In this photo, the sensor mote is attached to a peripheral nerve fiber of a rat. Credit:Ryan Neely/University of California at Berkeley
This week, a team at the University of California, Berkeley published a new wireless, implantable sensor that’s only three millimeters (about a tenth of an inch) in length. Aptly named “neural dust,” the device contains a piezoelectric crystal that converts ultrasonic vibrations from outside the body into electricity. This energy is then used to power a tiny transistor that contacts both the crystal and a nerve fiber.
When an impulse jolts through the nerve, it tweaks the circuits in the transistor, which in turn change the vibration of the crystal. These tiny flutters are then picked up by an ultrasound receiver and subsequently decoded. In this way, the device lets researchers closely monitor each spike of activity in a nerve.
Although the device is currently “read-only,” the team — not associated with Galvani — says that they are developing neural dust that can also stimulate nerves in a self-sustaining, closed-loop system.
Famm seems to welcome a healthy dose of competition in the nascent but burgeoning field.
“Clearly, open innovation … will be important,” he wrote back in 2013. Quoting the poet Cesare Pavese, Famm continued, “’If you wish to travel far and fast, travel light. Take off all your envies, jealousies, unforgiveness, selfishness and fears.’ Together we can bring about the era of electroceuticals.”
Banner image credit: GSK/YouTube
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles
This Light-Powered AI Chip Is 100x Faster Than a Top Nvidia GPU

How Scientists Are Growing Computers From Human Brain Cells—and Why They Want to Keep Doing It
These Brain Implants Are Smaller Than Cells and Can Be Injected Into Veins
What we’re reading