FDA OKs 1st Embryonic Stem Cell Trial for Geron, Stock Skyrockets

Share
The first clinical human trial using embryonic stem cells as a medical treatment in the United States has been approved by the U.S. Food and Drug Administration. Roughly ten patients will be treated about 2 weeks after they have suffered from acute spinal cord injuries that would normally result in permanent paralysis without the new therapy. The hope is that the stem cell therapy will help the body to heal itself from the spinal cord injury, allowing the patients to regain some or even most of their ability to walk and move.
In a landmark study published in 2005, this same therapy was shown to allow mice who were paralyzed as a result of an acute spinal cord injury to walk again. Should similar results be achieved in these human trials it will represent a major advance in efforts to heal what have historically been devastating and untreatable human injuries.
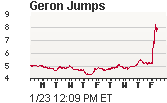
Geron, the company behind the trials, has a long history as a pioneer in scientific discovery. In 1999 Geron acquired the patents and intellectual property behind Dolly the lamb, the world sensation that was the first mammal to be cloned from an adult somatic cell, using the process of nuclear transfer. (wikipedia). In the stock market today, Geron's stock price surged in enthusiasm for the FDA approval. (Stock Chart: source). Geron has an entire website dedicated to the announcement which includes this very instructive video:
So how does this stem cell therapy from Geron work?
Contrary to common perception, stem cells are not what Geron will be injecting into the injured patients. Instead a different type of cells called oligodendrocyte progenitor cells will be derived from stem cells, and it is these progenitor cells that will be injected into the patients. Researchers at Geron are able to coax human embryonic stem cells (hESC) to differentiate into progenitor cells under specific laboratory conditions. According to Geron "these progenitor cells, once placed in the patient, have demonstrated remyelinating and nerve growth stimulating properties leading to restoration of function in animal models of acute spinal cord injury (Journal of Neuroscience, Vol. 25, 2005)."
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Officials from Geron are quick to caution against over optimistic expectations. Although the therapy has proven it can allow rats to regain their ability to walk after suffering an acute spinal cord injury, it is yet to be seen what effect this therapy will have on humans. Geron claims that their main objective is to prove the safety of stem cell based therapies, but we don't it. We all know that Geron is hoping that the therapy will restore motor function in humans similar to that seen in the rat trials.
It should be noted, as we have reported previously, that stem cell treatments outside of the United States have been commonplace for years. At first glance Geron's work therefore may not seem like a big deal, but this misses the point.
The Geron trials are a major step forward because they are the first ever approved by the FDA, bringing a whole new level of credibility, oversight, and scientific rigor to the field that has been lacking in other trials. Many of the other trials that have occurred outside of the jurisdiction of the FDA, such as those in China, have been ridiculed for having little oversight, questionable practices, and very little peer reviewed, reproducible data. In addition, many argue there is not much science behind these other efforts. The researchers literally seem to be injecting stuff into patients with a "lets see what happens" approach and with little basis of scientific theory. In stark contrast, the science behind Geron's work has been tested exhaustively in the lab on animals, the mechanism behind its operation is known, the patents backing their work are formidable, and the manufacture of the cells ideally meets strict demands for procedure, sterilization, and so forth.
Stem cell therapies have been a controversial issue in the United States because of ethical concerns surrounding the destruction of embryonic cells. For 8 years President Bush has severely limited the supply of stem cell lines that could be used by researchers and he has put a virtual lock on federal funding for related research. Officials are claiming that it is simply a coincidence that the FDA approved the trials just days after Bush left office and the stem cell friendly Obama stepped in, but is it really a coincidence? We aren't so sure. In any case, the rise of Obama portends a renewed openness in America for stem cell research and further hastens the coming era of miraculous treatments for the sick and injured.
Below is the Webcast Presentation Given to Investors earlier today:
Related Articles

More Space Junk Is Plummeting to Earth. Earthquake Sensors Can Track It by the Sonic Booms.

Researchers Break Open AI’s Black Box—and Use What They Find Inside to Control It

This Week’s Awesome Tech Stories From Around the Web (Through February 21)
What we’re reading