Talking With A Surgeon Who Implanted Telescopes in Eyes
Share
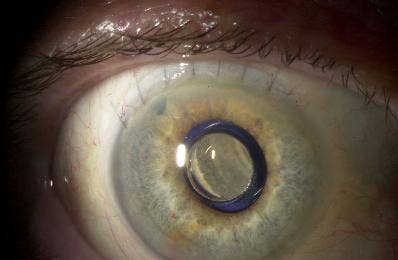
You may remember our earlier story on implantable miniature telescopes that helped treat blindness. Well, I had the good fortune of doing a follow-up interview with Dr. Kathryn Colby, Professor at Harvard Medical School and surgeon at Massachusetts Eye and Ear Infirmary. She was one of the primary surgeons who carried out the clinical trials with the telescope (the product name is CentraSight), and is an advisor to VisionCare, the company behind the technology. While the company is still waiting on FDA approval, Dr. Colby described to me some of the successes she's already seen.
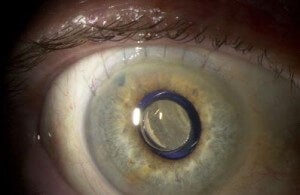
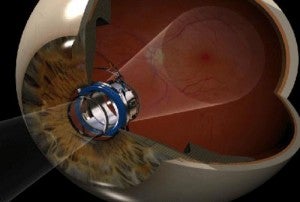
CentraSight is used to combat advanced macular degeneration (AMD). The condition causes a hole to form in the center of your vision, making you effectively blind. The implanted telescope expands the center field of vision so that the image can be captured on the part of the macula that is still healthy. AMD affects millions of Americans, and about 500,000 of these have cases advanced enough to benefit from CentraSight. According to VisionCare, that number will likely double by 2050. That means hundreds of thousands of people in the US alone may be able to see again with the telescope.
CentraSight's clinical trial results were good. Of the 206 patients 67% saw marked improvements in vision (3.5 lines on the eye chart or better) at the 1-year efficacy endpoint. On the National Eye Institute’s Visual Functioning Questionnaire, patients’ scores increased by meaningful amounts on 7 out of 8 relevant subscales. This demonstrates improvement in their quality of life. Results suggested that patients were less dependent on others, less worried or frustrated with their visual acuity, and better able to recognize facial expressions. The trial finished in 2004, and Dr. Colby says that most of the patients have maintained the benefits from the surgery and implant.

Dr. Colby also had a chance to collect some great anecdotal evidence. A sculptor who had to stop work because of her blindness could return to her studio. Another patient finally got back to bike riding. My personal favorite: one woman was thankful, but not as thankful as her cat, who had been accidentally kicked constantly after the woman had developed AMD.
Of course, there are some limitations to the clinical trials as well. The very nature of the telescope changes a patients vision forever. That means its a final solution to AMD, and will only be used on those patients within the end stages (near to total blindness). AMD is also age-related, so being at the end stage of AMD generally means being towards the end of one's life. Several patients have passed away in the five years since the trial ended. Dr. Colby seemed to think long term complications for the device were minimal, but its hard to tell when the lifetime of the product is longer than the lifetime of the patient.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Dr. Colby was quick to point out that implantation surgery was only one part of recovering one's eyesight. Centrasight adjusts the center field of vision to expand into the peripheral vision region. Only one eye is implanted (so the other eye can retain peripheral acuity) and a patient has to adjust to the new way of seeing. Any competent surgeon could give you CentraSight, but it takes a special kind of person to succeed with it. A 35 minute surgery is followed by three months of rehabilitation and therapy. Some people just won't have what it takes to get through that. According to Colby, potential patients should have a good attitude, motivation, and optimism.
What if you're crotchety and pessimistic? Implants aren't the only possible cure for blindness. Bionic eyes or stem cells might do the job as well. The big difference, as Colby points out, is that the implant has been tested and is ready now. Looking forward, advancements in AMD intervention (treatments, vitamin regimens, etc) may help combat the early stages of the disease and keep vision from degrading. Yet when it comes to end stage AMD, there really is just one choice. Right now it's implant or nothing. Or really...nothing, because the FDA is still waiting to give final approval.
The long wait for FDA approval is frustrating to those who wish to receive the implant. Colby says that her waiting list for the operation is lengthy. She's been telling them that FDA approval could come at any time, and it could, but she's had to say that for five years. It's a good sign that the advisory panel that met in March gave a unanimous vote for approval. Hopefully the bureaucracy will grind a little faster in the months to come.
[photos courtesy of VisionCare]
Related Articles

This Week’s Awesome Tech Stories From Around the Web (Through February 21)

What the Rise of AI Scientists May Mean for Human Research

This ‘Machine Eye’ Could Give Robots Superhuman Reflexes
What we’re reading