AI Can Now Design Proteins That Behave Like Biological ‘Transistors’
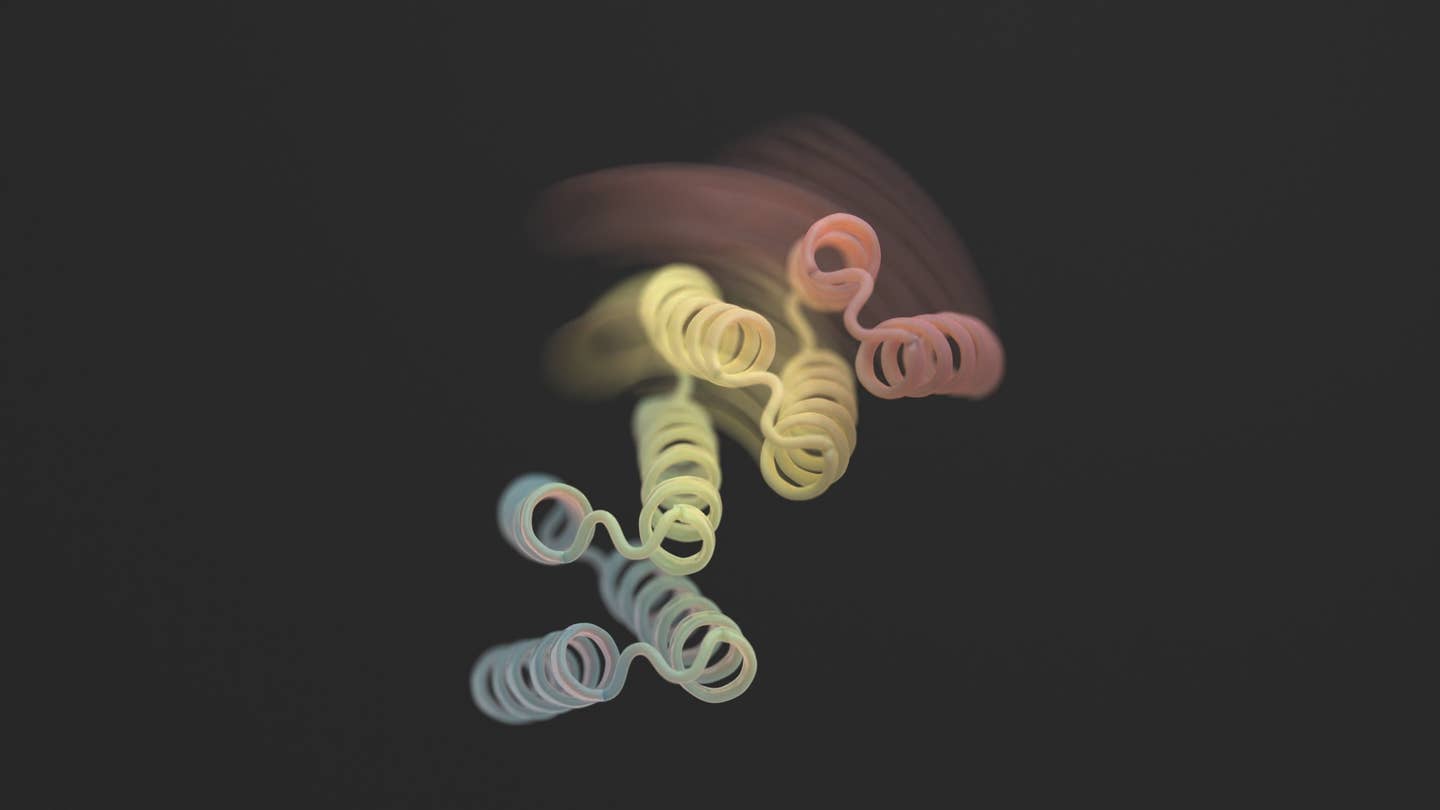
Share
We often think of proteins as immutable 3D sculptures.
That’s not quite right. Many proteins are transformers that twist and change their shapes depending on biological needs. One configuration may propagate damaging signals from a stroke or heart attack. Another may block the resulting molecular cascade and limit harm.
In a way, proteins act like biological transistors—on-off switches at the root of the body’s molecular “computer” determining how it reacts to external and internal forces and feedback. Scientists have long studied these shape-shifting proteins to decipher how our bodies function.
But why rely on nature alone? Can we create biological “transistors,” unknown to the biological universe, from scratch?
Enter AI. Multiple deep learning methods can already accurately predict protein structures—a breakthrough half a century in the making. Subsequent studies using increasingly powerful algorithms have hallucinated protein structures untethered by the forces of evolution.
Yet these AI-generated structures have a downfall: although highly intricate, most are completely static—essentially, a sort of digital protein sculpture frozen in time.
A new study in Science this month broke the mold by adding flexibility to designer proteins. The new structures aren’t contortionists without limits. However, the designer proteins can stabilize into two different forms—think a hinge in either an open or closed configuration—depending on an external biological “lock.” Each state is analogous to a computer’s “0” or “1,” which subsequently controls the cell’s output.
"Before, we could only create proteins that had one stable configuration," said study author Dr. Florian Praetorius at the University of Washington. "Now, we can finally create proteins that move, which should open up an extraordinary range of applications."
Lead author Dr. David Baker has ideas: “From forming nanostructures that respond to chemicals in the environment to applications in drug delivery, we're just starting to tap into their potential."
A Protein Marriage Made in AI
A quick bit of biology 101.
Proteins build and run our bodies. These macromolecules begin their journey from DNA. Genetic information is translated into amino acids, the building blocks of a protein—picture beads on a string. Each string is then folded into intricate 3D shapes, with some parts sticking to others. Called secondary structures, some configurations look like Twizzlers. Others weave into carpet-like sheets. These shapes further build on each other, forming highly sophisticated protein architectures.
By understanding how proteins gain their shapes, we can potentially engineer new ones from scratch, expanding the biological universe and creating new weapons against viral infections and other diseases.
Back in 2020, DeepMind’s AlphaFold and David Baker lab’s RoseTTAFold broke the structural biology internet by accurately predicting protein structures based on their amino acid sequences alone.
Since then, the AI models have predicted the shape of almost every protein known—and unknown—to science. These powerful tools are already reshaping biological research, helping scientists quickly nail down potential targets to combat antibiotic resistance, study the “housing” of our DNA, develop new vaccines or even shed light on diseases that ravage the brain, like Parkinson’s disease.
Then came a bombshell: generative AI models, such as DALL-E and ChatGPT, offered a tantalizing prospect. Rather than simply predicting protein structures, why not have AI dream up completely novel protein structures instead? From a protein that binds hormones to regulate calcium levels to artificial enzymes that catalyze bioluminescence, initial results sparked enthusiasm and the potential for AI-designed proteins seemed endless.
At the helm of these discoveries is Baker’s lab. Shortly after releasing RoseTTAFold, they further developed the algorithm to nail down functional sites on a protein—where it interacts with other proteins, drugs, or antibodies—paving the way for scientists to dream up new medications they haven’t yet imagined.
Yet one thing was missing: flexibility. A large number of proteins “code shift” in shape to change their biological message. The result could literally be life or death: a protein called Bax, for example, alters its shape into a conformation that triggers cell death. Amyloid beta, a protein involved in Alzheimer’s disease, notoriously takes a different shape as it harms brain cells.
An AI that hallucinates similar flip-flop proteins could edge us closer to understanding and recapitulating these biological conundrums—leading to new medical solutions.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Hinge, Line, and Sinker
Designing one protein at the atomic level—and hoping it works in a living cell—is hard. Designing one with two configurations is a nightmare.
As a loose analogy, think of ice crystals in a cloud that eventually form into snowflakes, each one different in structure. The AI’s job is to make proteins that can shift between two different “snowflakes” using the same amino acid “ice crystals,” with each state corresponding to an “on” or “off” switch. Additionally, the protein has to play nice inside living cells.
The team began with several rules. First, each structure should look vastly different between the two states—like a human profile standing or sitting. They could check this by measuring distances between atoms, explained the team. Second, the change needs to happen fast. This means the protein can’t completely unfurl before piecing itself back together into another shape, which takes time.
Then there are some groundskeeping guidelines for a functional protein: it needs to play nice with bodily liquids in both states. Finally, it has to act as a switch, changing its shape depending on inputs and outputs.
Meeting all “these properties in one protein system is challenging,” said the team.
Using a mix of AlphaFold, Rosetta, and proteinMPNN, the final design looks like a hinge. It has two rigid parts that can move relative to each other, while another piece remains folded. Normally the protein is closed. The trigger is a small peptide—a short chain of amino acids—that binds to the hinges and triggers its shape change. These so-called “effector peptides” were carefully designed for specificity, lowering their chances of grabbing onto off-target parts.
The team first added glow-in-the-dark trigger peptides to multiple hinge designs. Subsequent analysis found that the trigger easily grabbed onto the hinge. The protein’s configuration changed. As a sanity check, the shape was one previously predicted using AI analysis.
Additional studies using crystallized structures of the protein designs, either with or without the effector, further validated the results. These tests also hunted down design principles that made the hinge work, and parameters that tip one state to the other.
The take away? AI can now design proteins with two different states—essentially building biological transistors for synthetic biology. For now, the system only uses custom-designed effector peptides in their studies, which may limit research and clinical potential. But according to the team, the strategy can also extend to natural peptides, such as those that bind proteins involved in regulating blood sugar, regulate water in tissues, or influence brain activity.
“Like transistors in electronic circuits, we can couple the switches to external outputs and inputs to create sensing devices and incorporate them into larger protein systems,” the team said.
Study author Dr. Philip Leung adds: “This could revolutionize biotechnology in the same way transistors transformed electronics.”
Image Credit: Ian C Haydon/ UW Institute for Protein Design
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

What the Rise of AI Scientists May Mean for Human Research

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought
What we’re reading