Scientists Pump Up Lab-Grown Muscles for Robots With a New Magnetic Workout
Share
As I’m typing these words, I don’t think about the synchronized muscle contractions that allow my fingers to dance across the keyboard. Or the back muscles that unconsciously tighten to hold myself upright while sitting on a spongy cushion.
It’s easy to take our muscles for granted. But under the hood, muscle cells perfectly align to build fibers—interwoven with blood vessels and nerves—into a biological machine that lets us move about in our daily lives without a second thought.
Unfortunately, these precise cell arrangements are also why artificial muscles are difficult to recreate in the lab. Despite being soft, squishy, and easily damaged, our muscles can perform incredible feats—adapt to heavy loads, sense the outside world, and rebuild after injury. A main reason for these superpowers is alignment—that is, how muscle cells orient to form stretchy fibers.
Now, a new study suggests that the solution to growing better lab-grown muscles may be magnets. Led by Dr. Ritu Raman at the Massachusetts Institute of Technology (MIT), scientists developed a magnetic hydrogel “sandwich” that controls muscle cell orientation in a lab dish. By changing the position of the magnets, the muscle cells aligned into fibers that contracted in synchrony as if they were inside a body.
The whole endeavor sounds rather Frankenstein. But lab-grown tissues could one day be grafted into people with heavily damaged muscles—either from inherited diseases or traumatic injuries—and restore their ability to navigate the world freely. Synthetic muscles could also coat robots, providing them with human-like senses, flexible motor control, and the ability to heal after inevitable scratches and scrapes.
Raman’s work takes a step in that direction. Her team built a biomanufacturing platform focused on replicating the mechanical forces between muscle cells and their environment, a relationship essential for the cells to organize into tissues. But it’s not just about mimicking physical forces—stretching, pulling, or twisting. Rather, the platform also takes into account how mechanical movements alter communications between cells directing them to align.
Along with a custom algorithm, the platform essentially turned cells into a living, functional biomaterial that self-organizes and responds to pushes and pulls. In turn, the system could also shed light on muscle cells’ remarkable ability to adapt, align, and regenerate.
“The ability to make aligned muscle in a lab setting means that we can develop model tissues for understanding muscle in healthy and diseased states and for developing and testing new therapies that combat muscle injury or disease,” Raman said in a press briefing.
Muscling Through
Raman has long sought to use living cells as an adaptive biomanufacturing material.
Over a half decade ago, she engineered tiny 3D-printed cyborg bots with genetically-altered muscle cells that responded to light. Like moths to a flame, the bio-bots followed beams of light. Surprisingly, like well-trained athletes, the bots’ engineered muscles became more flexible as they exercised, allowing them to steer and rotate through different challenges.
The results made the team wonder: For lab-grown muscles to fully function, do we need to “exercise” the tissues?
The answer is seemingly yes. In a study last week, the team expanded upon their bio-bot results with light-activated muscle grafts to restore a large muscle injury in the hind legs of mice.
Muscle grafts are nothing new, but they need to integrate into their hosts, which is often difficult. Here, mice with Raman’s grafts completely recovered mobility in just two weeks.
The team shined beams of blue light on the muscle grafts daily as an exercise regime to strengthen them after implantation in the host. The workout didn’t just keep the grafts alive, it also spurred them to grow blood vessels and nerve cells that connected with the host body. With just half an hour a day for 10 days, the treatment boosted muscle force by three times.
“Exercising muscle grafts after they've implanted does more than just make muscle stronger, it also appears to affect how muscle communicates with other tissue, like blood vessels and nerves,” said Raman.
Yet a question remained: Can you make muscle fibers stronger with exercise in a petri dish outside the body?
Magnetic Workout
It’s not a crazy idea. Raman’s previous work found that zapping muscle fibers with electrical bursts for 30 minutes a day made the fibers stronger after just 10 days.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Muscles are wired to respond to electrical signals from the brain. However, they’re also sensitive to mechanical forces, which are hard to replicate in the lab.
“Generally, when people want to mechanically stimulate tissues in a lab environment, they grasp the tissue at both ends and move it back and forth, stretching and compressing the whole tissue,” Raman said in the briefing. “But this doesn't really mimic how cells talk to each other in our bodies.”
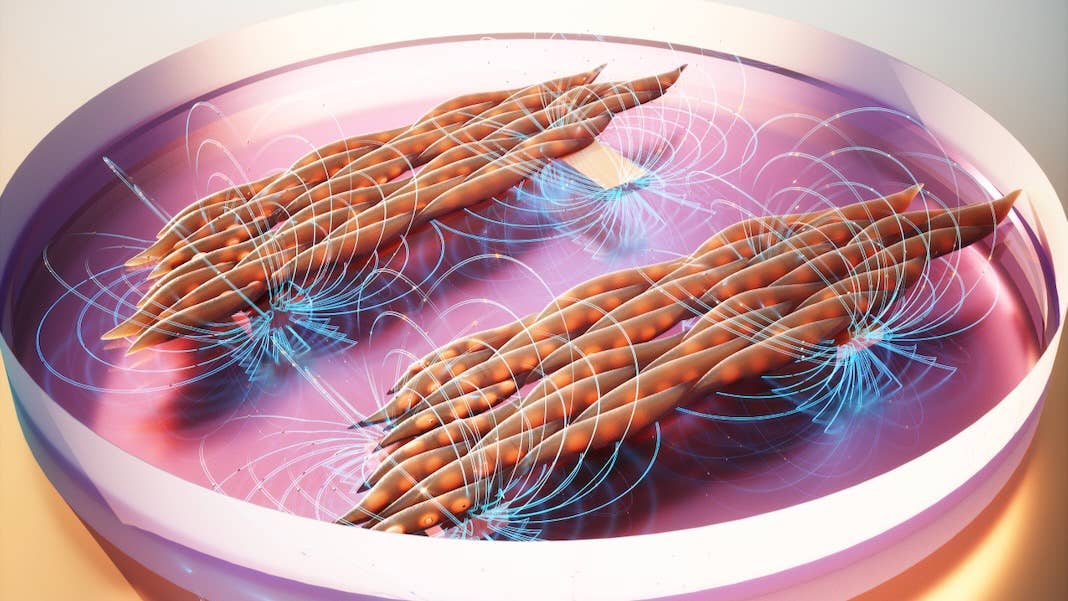
Enter MagMA. Short for magnetic matrix actuation (yeah, it’s a mouthful), the system mimics the gel-like structure surrounding muscle cells. It’s basically a sandwich: The inner layer is a magnetic silicone “filling” embedded with iron microparticles. The two “bread slices” consist of a hydrogel made with ingredients from a natural protein.
Added to the bottom of a petri dish, MagMA functions like the gel-like matrix that muscle cells interact with—but with a magnetic boost.
The team wanted to know if, like electricity and light, magnets can also be used to exercise muscles and encourage them to form fibers.
They grew a layer of muscle cells in a MagMA-coated petri dish. By sliding a magnet across the petri dish, they moved the magnetic particles in the hydrogel, which in turn mechanically flexed the cells. The system is highly controllable: changing the magnet’s path alters the magnitude and direction of the mechanical forces the cells experience, in turn changing their orientation.
Overall, the team found the muscle cells rapidly aligned into neatly bundled muscle fibers. Although the fibers weren’t stronger, they were more organized. In contrast, cells grown in standard dishes grew chaotically, forming muscle fibers at odds with their neighbors’ orientation.
The difference in structure altered the muscle’s function: muscle cells programmed with MagMA hydrogels twitched together in synchrony—a critical need when engineering functional muscles. Those grown without MagMA also moved, but with each fiber twitching to its own tune, resulting in a sort of spasm.
“We were very surprised by the findings of our study,” said Raman. “This confirmed our understanding that the form and function of muscle are intrinsically connected, and that controlling form can help us control function.”
The magnetic platform is still in its early days. The team is now looking to optimize magnet strength and other parameters to best stimulate muscle growth and function. They’re also expanding the platform to other cell types in tissue engineering and tailoring the hydrogel component with other materials for specific biomanufacturing needs.
The team is certain that building muscles is just the first step. We think “this platform will drive a broad range of fundamental and translational studies of mechanobiology, regenerative medicine, and biohybrid robotics,” they wrote in the study.
Image Credit: Ella Marushchenko
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

This ‘Machine Eye’ Could Give Robots Superhuman Reflexes

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought
What we’re reading
