Scientists Say They Extended Mice’s Lifespans 25% With an Antibody Drug
Share
Age catches up with us all. Eyes struggle to focus. Muscles wither away. Memory dwindles. The risk of high blood pressure, diabetes, and other age-related diseases skyrockets.
A myriad of anti-aging therapies are in the works, and a new one just joined the fray. In mice, blocking a protein that promotes inflammation in middle age increased metabolism, lowered muscle wasting and frailty, and reduced the chances of cancer.
Unlike most previous longevity studies that tracked the health of aging male mice, the study involved both sexes, and the therapy worked across the board.
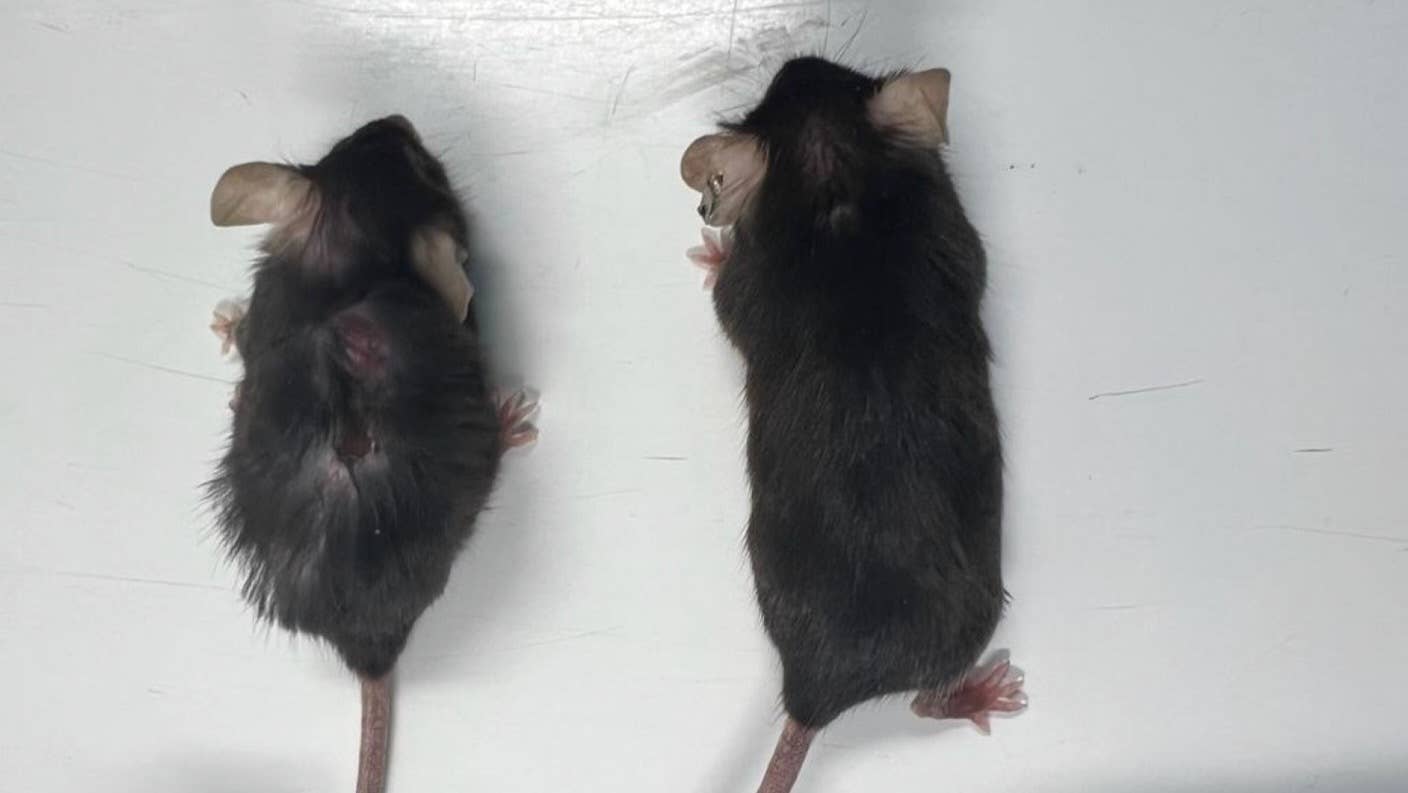
Lovingly called “supermodel grannies” by the team, the elderly lady mice looked and behaved far younger than their age, with shiny coats of fur, less fatty tissue, and muscles rivaling those of much younger mice.
The treatment didn’t just boost healthy longevity, also known as healthspan—the number of years living without diseases—it also increased the mice’s lifespan by up 25 percent. The average life expectancy of people in the US is roughly 77.5 years. If the results translate from mice to people—and that’s a very big if—it could mean a bump to almost 97 years.
The protein, dubbed IL-11, has been in scientists’ crosshairs for decades. It promotes inflammation and causes lung and kidney scarring. It’s also been associated with various types of cancers and senescence. The likelihood of all these conditions increases as we age.
Among a slew of pro-aging proteins already discovered, IL-11 stands out as it could make a beeline for testing in humans. Blockers for IL-11 are already in the works for treating cancer and tissue scarring. Although clinical trials are still ongoing, early results show the drugs are relatively safe in humans.
"Previously proposed life-extending drugs and treatments have either had poor side-effect profiles, or don't work in both sexes, or could extend life, but not healthy life, however this does not appear to be the case for IL-11," said study author Dr. Stuart Cook in a press release. “These findings are very exciting.”
Strange Coincidence
In 2017, Cook zeroed in on IL-11 as a treatment target for heart and kidney scarring, not longevity. Injecting IL-11 triggered the conditions, eventually leading to organ failure. Genetically deleting the protein protected against the diseases.
It’s easy to call IL-11 a villain. But the protein is an essential part of the immune system. Produced by the bone marrow, it’s necessary for embryo implantation. It also helps certain types of blood cells grow and mature, notably those that stop bleeding after a scrape.
With age, however, the protein tends to goes rogue. It sparks inflammation across the body, damaging cells and tissues and contributing to cancer, autoimmune disorders, and tissue scarring. A “hallmark of aging,” inflammation has long been targeted as a way to reduce age-related diseases. Although IL-11 is a known trigger for inflammation, it hasn’t been directly linked to aging.
Until now. The story is one of chance.
“This project started back in 2017 when a collaborator of ours sent us some tissue samples for another project,” said study author Anissa Widjaja in the press release. She was testing a method to accurately detect IL-11. Several samples of an old rat’s proteins were in the mix, and she realized that IL-11 levels were far higher in the samples than in those from younger mice.
“From the readings, we could clearly see that the levels of IL-11 increased with age, and that’s when we got really excited,” she said.
Longevity Blocker
The results spurred the team to shift their research focus to longevity. A series of tests confirmed IL-11 levels consistently rose in a variety of tissues—muscle, fat, and liver—in both male and female mice as they aged.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


To see how IL-11 influences the body, the team next deleted the gene coding for IL-11 and compared mice without the protein to their normal peers. At two years old, considered elderly for mice, tissues in normal individuals were littered with genetic signatures suggesting senescence—when cells lose their function but are still alive. Often called “zombie cells,” they spew out a toxic mix of inflammatory molecules and harm their neighbors. Elderly mice without IL-11, however, had senescence genetic profiles similar to those of much younger mice.
Deleting IL-11 had other perks. Weight gain is common with age, but without IL-11, the mice maintained their slim shape and had lower levels of fat, greater lean muscle mass, and shiny, full coats of fur. It’s not just about looks. Cholesterol levels and markers for liver damage were far lower than in normal peers. Aged mice without IL-11 were also spared shaking tremors—otherwise common in elderly mice—and could flexibly adjust their metabolism depending on the quantity of food they ate.
The benefits also showed up in their genetic material. DNA is protected by telomeres—a sort of end cap on chromosomes—that dwindle in length with age. Ridding cells of IL-11 prevented telomeres from eroding away in the livers and muscles of the elderly mice.
Genetically deleting IL-11 is a stretch for clinical use in humans. The team next turned to a more feasible alternative: An antibody shot. Antibodies can grab onto a target, in this case IL-11, and prevent it from functioning.
Beginning at 75 weeks, roughly the equivalent of 55 human years, the mice received an antibody shot every month for 25 weeks—over half a year. Similar antibodies are already being tested in clinical trials.
The health benefits in these mice matched those in mice without IL-11. Their weight and fat decreased, and they could better handle sugar. They also fought off signs of frailty as they aged, experiencing minimal tremors and problems with gait and maintaining higher metabolisms. Rather than wasting away, their muscles were even stronger than at the beginning of the study.
The treatment didn’t just increase healthspan. Monthly injections of the IL-11 antibody until natural death also increased lifespan in both male and female mice by up to 25 percent.
“These findings are very exciting. The treated mice had fewer cancers and were free from the usual signs of aging and frailty... In other words, the old mice receiving anti-IL-11 were healthier,” said Cook.
Although IL-11 antibody drugs are already in clinical trials, translating these results to humans could face hurdles. Mice have a relatively short lifespan. A longevity trial in humans would be long and very expensive. The treated mice were also contained in a lab setting, whereas in the real world we roam around and have differing lifestyles—diet, exercise, drinking, smoking—that could confound results. Even if it works in humans, a shot every month beginning in middle age would likely rack up a hefty bill, providing health and life extension only to those who could afford it.
To Cook, rather than focusing on extending longevity per se, tackling a specific age-related problem, such as tissue scarring or losing muscles is a better alternative for now.
“While these findings are only in mice, it raises the tantalizing possibility that the drugs could have a similar effect in elderly humans. Anti-IL-11 treatments are currently in human clinical trials for other conditions, potentially providing exciting opportunities to study its effects in aging humans in the future,” he said.
Image Credit: MRC LMS, Duke-NUS Medical School
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles
Single Injection Transforms the Immune System Into a Cancer-Killing Machine
New Gene Drive Stops the Spread of Malaria—Without Killing Any Mosquitoes

New Immune Treatment May Suppress HIV—No Daily Pills Required
What we’re reading