New Studies Show Cells From Fetus End Up In Mothers’ Brains And Hearts

Share
Microchimerism results when cells from two genetically distinct populations appear in the same tissue, organ, or individual, such as occurs during an organ transplant or during pregnancy. It has been known for some time that, during pregnancy, fetal cells end up circulating within the mother’s bloodstream. A relatively new discovery, however, is that these fetal cells don’t just remain in the blood stream but travel to organs such as the heart or brain.
Until recently fetal microchimeric cells which traveled to the maternal brain had only been seen in mice. But a new study shows that microchimeric cells occur in humans as well.
To detect the microchimeric cells in the maternal brain, scientists at the Fred Hutchinson Cancer Research Center in Seattle performed PCR analysis on tissue from 59 autopsied female brains. They found in 63 percent of the brains genes that are unique to the Y-chromosome, indicating they originated from a male, thus, microchimeric cells from a male fetus the women had given birth to at some point in their lives. Microchimeric cells persist in the maternal blood stream for years after pregnancy.
What’s fascinating about these fetal cells is that they resemble pluripotent stem cells – they have the ability to become heart or brain cells. What this means functionally is still uncertain. But the potential for these so-called fetal microchimeric cells to incorporate and actually help repair maternal tissue is a new and exciting area of medical research.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


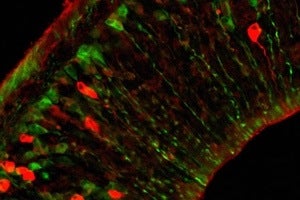
By labeling cells from a mouse fetus green, they were able to detect them in the maternal brain. Now scientists have also shown that fetal microchimeric cells show up in human maternal brains as well. [Image: Harvard University]
Previous research in rats has shown microchimeric cells from the fetus migrating to the maternal heart which had been injured. A kind of trans-placental stem cell transplant, the fetal cells selectively targeted the injured area where they differentiated into several types of cardiac cells and aided in its repair. Another study in mice showed fetal microchimeric cells migrated to the maternal brainwhere they became neurons. Furthermore, more of the cells moved in to areas after they’d been damaged. Although it appears as though the fetal cells might be playing the regenerative role that has long been hoped for stem cell, it still remains to be shown whether or not the new neurons are being functionally incorporated into the maternal brain.
But, like the child from which they originate, microchimeric cells have the potential to make the mother’s life both easier, and more difficult. Studies have linked the prolonged presence of the fetal cells in the mother’s blood to autoimmune disease. It is thought that the immature immune cells from the fetus stimulate inflammation and bring about autoimmune disease, such as in graft-versus-host disease in which immune cells from donated tissue attacks the host’s immune system. One study showed that fetal microchimeric cells were higher in women with multiple sclerosis, an autoimmune disease, compared to their siblings without multiple sclerosis. Some think that the increase in autoimmune disease among women over the past 40 years is due to the relationship between microchimeric cells, autoimmune disease, and abortion. Because the placenta is destroyed, an abortion results in a higher transfer of mircochimeric cells to the maternal bloodstream.
Further research is needed to elucidate the role of these interesting cells in the mother’s physiology. And perhaps, when more is known, they can be manipulated to either foster tissue repair or decrease the chance for autoimmune disease. From a scientific perspective, the bond between mother and child just got stronger, and even more complex.
Peter Murray was born in Boston in 1973. He earned a PhD in neuroscience at the University of Maryland, Baltimore studying gene expression in the neocortex. Following his dissertation work he spent three years as a post-doctoral fellow at the same university studying brain mechanisms of pain and motor control. He completed a collection of short stories in 2010 and has been writing for Singularity Hub since March 2011.
Related Articles

This Week’s Awesome Tech Stories From Around the Web (Through February 21)

What the Rise of AI Scientists May Mean for Human Research

This ‘Machine Eye’ Could Give Robots Superhuman Reflexes
What we’re reading
