Athersys Secures Stem Cell Patents and Stock Swells

Share
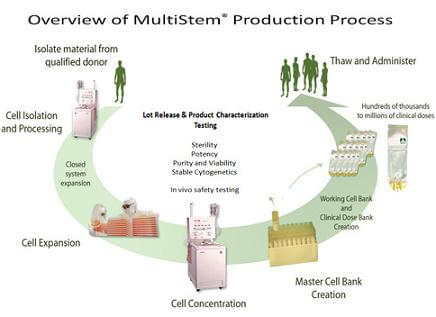
Biopharmaceutical company Athersys (NASDAQ: ATHX) received a major boost in its stock price on February 10th, following the announcement that it had received US and European patents related to its MultiStem technology. Guess there's nothing investors like more than learning your company has dibs on stem cells. MultiStem is a product that is essentially non-embryonic multipotent stem cells derived from the bone marrow of select human donors. Athersys is able to take these donated stem cells, isolate them, grow them, and then process them so they can be used in patients. A single donor could provide enough MultiStem doses for hundreds of thousands if not millions of recipients! Just as impressive, no immunosuppression or donor matching is needed when using MultiStem. Athersys is hoping to make their product the stem cell equivalent of Type O blood and is working with Pfizer, Angiotech and others to develop specific treatments. Let's hope Athersys's bump in stock price is a good sign that safe and effective stem cell treatments are coming to the mainstream soon.
Athersys's price jump was pronounced. Stocks rose $0.80 to around $3.60 in the opening of February 10th. They've since come back down to around $3.30 as of the time of writing. As impressive as that increase may be, it pales compared to the $4.50 increase up to $5.50 Athersys saw in December of 2009. That jump occurred just after the announced partnership with Pfizer to develop a MultiStem treatment for inflammatory bowel disease. The lesson here: investors may love proprietary technology, but they love the application of that technology even more. Oh, and attaching yourself to a pharmaceutical giant ain't bad either.
I'm floored at the idea that a stem cell product could develop into a universal pharmaceutical. Of course, the discovery of Type O blood as a universal donor could have been just as astounding in its time. Most other stem cell treatments we've seen have either been between matched donors, or through analogous infusions - where a patient's own stem cells are used. The processing of such stem cells takes time, and while we've heard of some amazingly quick responses to treatments, I would imagine that hospitals will prefer a single product they can administer to any patient without the need for selection tests or other prep work.
Not only is MultiStem able to work in many different patients, Athersys believes it could work for many different illnesses. They claim that the stem cell product could help treat acute myocardial infarction (in development with Angiotech), inflammatory bowel disease (in development with Pfizer), bone marrow transplants, and stroke among others.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


As with many stem cell treatments, it's not exactly clear how MultiStem enabled programs work to fight these diseases. Athersys claims the multipotent stem cells have a "drug-like profile" that works by regulating the immune system, protecting cells, and promoting repair. They also say that the stem cells are mostly (or completely) cleared from the body over time. Considering the peer reviewed work and publications on the Athersys site, I'm inclined to believe in MultiStem's potency even if the way in which it works seem very broad.
I do worry, however, whenever I hear about the patenting of human cells. Owning life has a bad history, obviously. Athersys holds 14 patents and has another 120 pending based on its technologies. The most recent Athersys patents focus on the process of developing MultiStem doses but there is an implicit argument for the intellectual property extending into the donor cells themselves because they must be carefully screen and selected. Of course, successfully isolating and expanding stem cells takes expertise, and the fact that Athersys can could possibly create millions of doses from a single donor is truly extraordinary. I don't begrudge them the profits and successes they are likely to reap if MultiStem becomes the blockbuster drug it could be, though I wonder what kind of compensation donors receive.
We'll know more once MultiStem has been leveraged into successful treatments, but Athersys could be onto a really huge medical development here. Imagine a stem cell therapy that simply involved your doctor going into a freezer, thawing some MultiStem and administering it intravenously. That's incredible. As with many stem cell treatments, we're likely to face years of clinical trials and experimentation before the technology is widely available for use. Still, I have high hopes that the investment interest in Athersys means that the world is ready and willing to give stem cells a chance.
[image credit: Athersys]
Related Articles

Researchers Break Open AI’s Black Box—and Use What They Find Inside to Control It

This Week’s Awesome Tech Stories From Around the Web (Through February 21)

What the Rise of AI Scientists May Mean for Human Research
What we’re reading