Zombie Cells Have a Weakness. An Experimental Anti-Aging Therapy Exploits It.
Share
Senescent cells are biochemical waste factories.
A new study suggests that a way to wipe them out is a medicine already approved for eye problems.
Dubbed “zombie cells,” senescent cells slowly accumulate with age or with cancer treatments. The cells lose their ability to perform normal functions. Instead, they leak a toxic chemical soup into their local environment, increasing inflammation and damaging healthy cells.
Over a decade of research has shown eliminating these cells with genetic engineering or drugs can slow down aging symptoms in mice. It's no wonder investors have poured billions of dollars into these “senolytic” drugs.
There are already hints of early successes. In one early clinical trial, cleaning out zombie cells with a combination of drugs in humans with age-related lung problems was found to be safe. Another study helped middle-aged and older people maintain blood pressure while running up stairs. But battling senescent cells isn’t just about improving athletic abilities. Many more clinical trials are in the works, including strengthening bone integrity and combating Alzheimer’s.
But to Carlos Anerillas, Myriam Gorospe, and their team at the National Institutes of Health (NIH) in Baltimore, therapies have yet to hit zombie cells where it really hurts.
In a study in Nature Aging, the team pinpointed a weakness in these cells: They constantly release toxic chemicals, like a leaky nose during a cold. Called SASP, for senescence-associated secretory phenotype, this stew of inflammatory molecules contributes to aging.
Lucky for us, this constant release of chemicals comes at a price. Zombie cells use a “factory” inside the cell to package and ship their toxic payload to neighboring cells and nearby tissues. All cells have these factories. But the ones in zombie cells go into overdrive.
The new study nailed down a protein pair that’s essential to the zombie cells’ toxic spew and found an FDA-approved drug that inhibits the process. When given to 22-month-old mice—roughly the human equivalent of 70 years old—they had better kidney, liver, and lung function within just two months of treatment.
The work “stands out,” said Yahyah Aman, an editor at Nature Aging. It’s an “exciting target for new senolytic drug development,” added Ming Xu at UConn Health, who wasn’t involved in the study.
A Molecular Metropolitan
Each cell is a bustling city with multiple neighborhoods.
Some house our genetic archives. Others translate those DNA codes into proteins. There are also acid-filled dumpsters and molecular recycling bins to keep each cell clear of waste.
Then there’s the ER. No, not the emergency room, but a fluffy croissant-like structure. Called the endoplasmic reticulum, it’s Grand Central for new proteins. The ER packages proteins and delivers them to internal structures, the cell’s surface, or destinations outside the cell.
These “secretory” packages are powerful regulators that control local cellular functions. Normally, the ER helps cells coordinate their responses with neighboring tissues—say, allowing blood to clot after a scrape or stimulating immune responses to heal the damage.
Senescent cells hijack this process. Instead of productive signaling, they instead release a toxic soup of chemicals. These cells aren’t born harmful. Rather, they’re transformed by a lifetime of injury—damage to their DNA, for example. Faced with so much damage, normal cells would wither away, allowing healthy new cells to replace them in some tissues like the skin.
Zombie cells, in contrast, refuse to die. As long as the harm stays below a lethal level, the cells live on, expelling their deadly brew and harming others in the vicinity.
These traits make zombie cells a valuable target for anti-aging therapies. And there have been promising treatments. Most have relied on existing knowledge or ideas about how zombie cells work. Researchers then seek out chemicals in massive drug libraries that might disrupt their function. While useful, this strategy can miss treatment options.
The new study went rogue. Rather than starting out with hypotheses, they screened the whole human genome to find new vulnerabilities.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


A Wild West
In their hunt, the team turned to CRISPR. Famously known as a gene editor, CRISPR is now often used to pinpoint genes and proteins that contribute to cellular functions. Here, the team disrupted every gene in the human genome to pinpoint those that eliminated zombie cells.
Their work paid off. The screen found a protein pair critical for senescent cell survival. The team next looked for an FDA-approved drug to disrupt the pair. They found what they were looking for in verteporfin, a drug approved to treat eye blood vessel disease.
In several zombie cell cultures with the protein pair, the drug drove senescent cells into apoptosis—that is, the “gentle falling of the leaves,” a sort of cell death does no harm to surrounding cells.
Digging deeper, the drug seemed to directly target the zombie cells’ endoplasmic reticulum—their shipping center. Cells treated with the drug couldn’t sustain the delicate multi-layered structure, and it subsequently shriveled into a shape like a wet, crumpled paper towel.
“A shrunken ER triggered a metabolic crisis” in zombie cells, explained Anerillas and Gorospe. It “culminated with their death.”
Ageless Mice
As a proof of concept, the team injected elderly mice—roughly the age of a 70-year-old human—with verteporfin once a month for two months.
In just a week, mice treated with verteporfin showed fewer molecular signs of senescence in their kidney, liver, and lungs. Their fur was more luxurious compared to control mice without the drug.
As we age, immune cells often enter the lungs and cause damage. Verteporfin nixed this infiltration and reduced lung scarring in mice—which is often linked to decreased breathing capacity. Similarly, according to blood tests, the drug also helped restore function in the mice’s kidneys and liver.
Decreased numbers of senescent cells dampened inflammatory signals, which could explain the rejuvenating effects, explained the team. Verteporfin also stopped a “guardian” protein that protects senescent cells from death, further triggering their demise.
Tapping into a zombie cell’s unique vulnerabilities is a new strategy in the development of senolytics. There’s far more to explore. The endoplasmic reticulum isn’t the only cell component in the biological waste factory. Other cellular components that generate senescent cell poisons could also be blocked and help remove the cells themselves.
It’s a promising alternative to existing methods for wiping out senescent cells. The strategy could “greatly expand the catalog of senolytic therapies,” the team wrote.
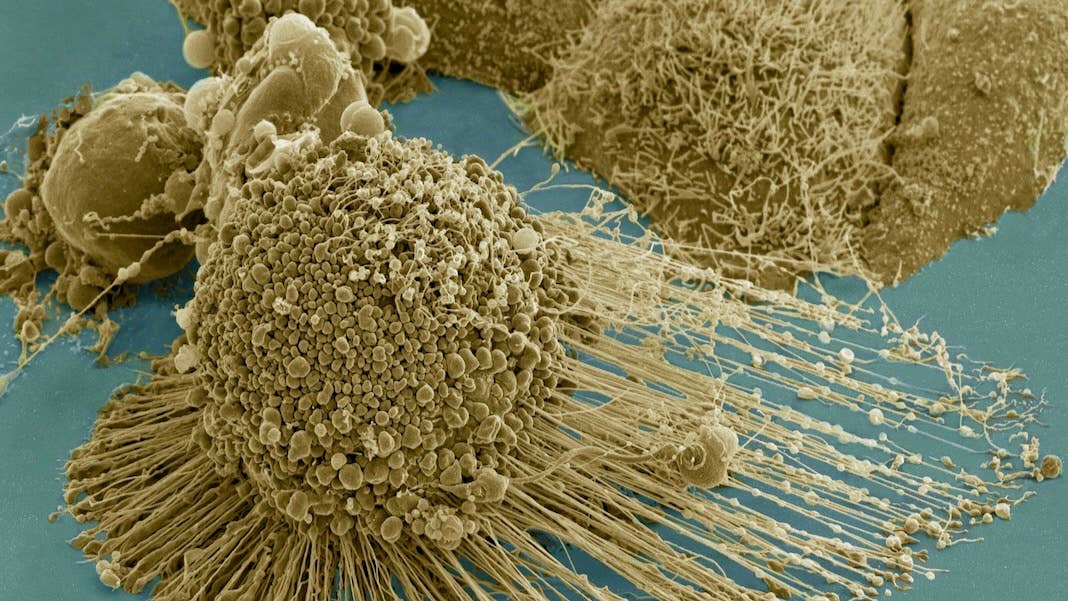
Image Credit: A HeLA cell undergoing apoptosis. Tom Deerinck / NIH / FLICKR
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles
These Supercharged Immune Cells Completely Eliminated Solid Tumors in Mice

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought
What we’re reading