Humans and bacteria are in a perpetual war.
For most of history, bacteria won. Before 1928, a simple scrape on the knee, a cut when cooking dinner, or giving birth could lead to death from infection.
The discovery of penicillin, a molecule secreted from mold, changed the balance. For the first time, humans had a way to fight back. Since then, generations of antibiotics have targeted different phases of bacterial growth and spread inside the body, efficiently eliminating them before they can infect other people.
But bacteria have an evolutionary upper hand. Their DNA readily adapts to evolutionary pressures—including from antibiotics—so they can mutate over generations to escape the drugs. They also have a “phone line” of sorts that transmits adapted DNA to other nearby bacteria, giving them the power to resist an antibiotic too. Rinse and repeat: Soon an entire population of bacteria gains the ability to fight back.
We might be slowly losing the war. Antibiotic resistance is now a public health threat that caused roughly 1.27 million deaths around the globe in 2019. The World Health Organization (WHO) and others say that without newer generations of antibiotics, surgery, cancer chemotherapy, and other life-saving treatments face increasing risk of death due to infection.
Traditionally, a new antibiotic takes roughly a decade to develop, test, and finally reach patients.
“There is an urgent need for new methods for antibiotic discovery,” Dr. Luis Pedro Coelho, a computational biologist and author of a new study on the topic, said in a press release.
Coelho and team tapped into AI to speed up the whole process. Analyzing huge databases of genetic material from the environment, they uncovered nearly one million potential antibiotics.
The team synthesized 100 of these AI-discovered antibiotics in the lab. When tested against bacteria known to resist current drugs, they found 63 readily fought off infections inside a test tube. One worked especially well in a mouse model of skin disease, destroying a bacterial infection and allowing the skin to heal.
“AI in antibiotic discovery is now a reality and has significantly accelerated our ability to discover new candidate drugs. What once took years can now be achieved in hours using computers,” said study co-senior author Dr. César de la Fuente at Penn Medicine in another press release.
Antibiotic Adversary
It’s easy to take antibiotics for granted. Say you have an ear infection from always wearing wireless earbuds. You get a prescription, dab it in, and all goes well.
Or does it? With time, the drops could potentially struggle to hold the infection back. This “antibiotic resistance” is key in the evolutionary battle between bacteria and humanity.
Antibiotics usually work to stop bacteria from replicating multiple ways. Like human cells, bacterial cells have a cell wall, a wrapper that keeps DNA and other biological components inside. One type of antibiotic destroys the wall, preventing the pathogen from spreading. Others target genetic material or inhibit metabolic pathways necessary for the bacteria to survive.
Every one of these strategies has taken decades of research to uncover and develop into medicine. But microbes rapidly mutate. Some bacteria, for example, develop “pumps” on their surfaces that literally throw out the drugs. Others evolve enzymes that shut down antibiotics by slightly changing their protein target sites through DNA mutation, neutering their effect.
Each strategy, by itself, is hard to evolve. But bacteria have another trick up their sleeves—horizontal transfer. Here, antibiotic-resistant genes are encoded into small circular pieces of DNA that can transfer to neighboring cells through a biological “highway”—a physical tube—endowing the recipients with a similar ability to fight off antibiotics.
Finding a way to kill off invading bacteria is tough. If bacteria evolve to evade that target, then the antibiotic and other chemically similar ones rapidly lose their effect. So, is there a way to find antibiotics that bacteria—or even nature itself—have never seen before?
An AI Solution
AI is beginning to revolutionize biology. From predicting protein structures to designing antibodies, these algorithms are tackling some of humanities’ most severe health disorders.
Traditionally, searching for antibiotics has mostly been trial-and-error, with scientists often scraping samples from exotic mosses or other sources that could potentially fight off infections.
In the new study, the team aimed to find new versions of a type of antibiotic based on antimicrobial peptides (AMPs). Similar to proteins, these are made of relatively short strings of molecules called amino acids. The peptides are found across the living world and can disrupt microbial growth by breaking down cell walls and causing bacteria to “explode.” They’ve already been used clinically as antimicrobial drugs and are currently being tested in clinical trials for yeast infections. However, like other antibacterials, they run the risk of resistance.
As the discovery of penicillin suggested nearly 100 years ago, the natural world is a bountiful source of potential antibiotics. In the study, the team used machine learning to look for antimicrobial peptides with possible antibiotic properties in over 63,000 publicly available metagenomes—genetic information isolated from multiple organisms in an environment—and nearly 88,000 high-quality microbial genomes. The sources came from across the globe, ocean and land, and also contained human and animal gut microbes. These data were merged into the AMPSphere database, which is open for anyone to explore.
The resource allowed scientists to mine the “entirety of the microbial diversity that we have on Earth—or a huge representation of that—and find almost one million new molecules encoded or hidden within all that microbial dark matter,” de la Fuente told The Guardian.
To test their findings, the team pulled out 100 candidates and synthesized them in the lab. In test tubes, 79 disrupted cell membranes, and 63 completely killed off at least one of the dangerous bugs.
“In some cases, these molecules were effective against bacteria at very low doses,” said de la Fuente.
The team next developed an antibiotic peptide from the database to tackle a dangerous bug causing skin lesions in mice. With just one shot, the AI-discovered drug inhibited bacterial growth, and the mice didn’t appear to suffer side effects based on body weight measurements.
“We have been able to just accelerate the discovery of antibiotics,” de la Fuente told The Guardian. “So instead of having to wait five, six years to come up with one candidate, now, on the computer, we can, in just a few hours, come up with hundreds of thousands of candidates.”
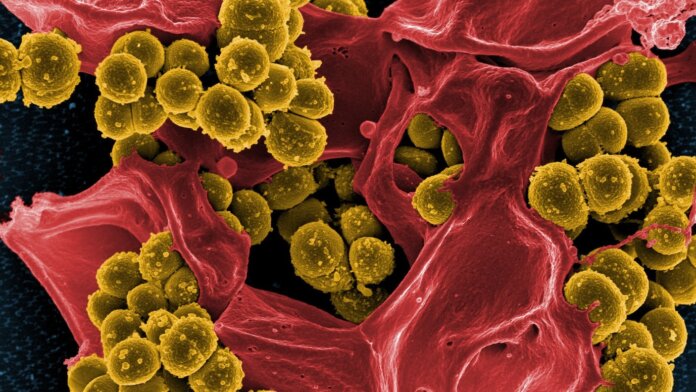
Image Credit: Antibiotic-resistant staph (yellow) and a dead white blood cell (red). National Institute of Allergy and Infectious Diseases (NIAID)/NIH