Implant that Shocks Brain to Treat Epilepsy in Clinical Trials
Share
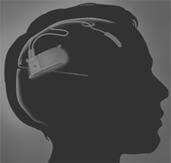
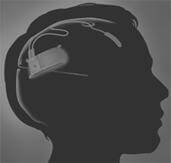
The medical uses of electrically shocking your brain have a dubious history and a worse portrayal in movies. One Flew Over A Cuckoo's Nest certainly didn't leave viewers rushing to strap electrodes to their head. Yet, the medical industry continues to explore how mild electrical stimulation could help with brain diseases the same way that pacemakers help with heart disease. Neuropace, a company based in Silicon Valley, is in clinical trials with their RNS System. The RNS is a neurostimulator implant that monitors brain activity and shocks select regions to prevent epileptic seizures. Currently in clinical trial, the device represents a new era of healing the brain through electric shock.
Epilepsy affects around 1% of the world's population (2.5 million or so in the US alone) and can be treated with medications or even surgeries. However, some patients are not good candidates for the epilepsy surgery, and many (Neuropace estimates 40-50%) are dissatisfied with their medications due to side effects or lack of efficacy. The RNS implant could help millions worldwide control or prevent their epileptic seizures. Similar treatments with the same implant could be used for many other neural illnesses.
The concept behind the RNS implant is treating seizures through 'responsive stimulation', which is based on work done by Bergey et al in 2002 (Epilepsia Vol 43, Issue s7). Regions of the brain before a seizure demonstrate erratic signals that can be stopped using appropriate electrical stimulation. Most other anti-epileptic systems created up to now do not monitor the brain for activity. Instead, they simply shock the brain on a regular basis as prevention. RNS is monitoring more and shocking less. That's a philosophy I can get behind.
In order to provide that stimulation, the RNS implant is placed under the skull by a surgeon and electrodes are connected to the relevant portions of the brain. Programming and data acquisition is conducted wirelessly using a wand connected to a modified laptop. This seems somewhat like the BrainGate 2 implant we discussed earlier.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Neuropace's clinical trials are very rigorous and will have taken a total of 2- 3 years to complete. Prospective patients keep seizure diaries for 3-15 months, and undergo close scrutiny before being approved. Once implanted, the patient undergoes 5 months of double blind testing. Termed 'sham stimulation', some patients will have their implants on for that period of time, and some will have implants turned off without their knowing. To insure a double blind study one of the monitoring doctors will also be unaware if the patient is actually receiving shocks. After the sham stimulation, all patients will have their RNS implants activated.

The trials involve more than 240 patients in 28 facilities across the US (including Yale Medical, Johns Hopkins, and Mayo Clinics) and should be finished around December 2009. It is targeting only those patients over 18 years old who show poor response to at least two epilepsy medications. The patients will stay on those meds during the trial. Preliminary studies suggest the RNS implant is effective in treating seizures.
One day, having a brain implant could be seen as no more extraordinary than having a pacemaker. Regulation of destructive brain signals (such as epileptic seizures) or controlling machines (as with Braingate) will be just some of the possible applications. The work done with these implants may pave the way for input/output devices using brain electrical signals. We could even see neural enhancement through measured stimulation. Of course, anyone thinking about tinkering with the mind via electrical shocks should first take a long hard look at Jack Nicholson.
Related Articles

Sparks of Genius to Flashes of Idiocy: How to Solve AI’s ‘Jagged Intelligence’ Problem

Researchers Break Open AI’s Black Box—and Use What They Find Inside to Control It

What the Rise of AI Scientists May Mean for Human Research
What we’re reading