The Incredible Regenerating Mouse
Share
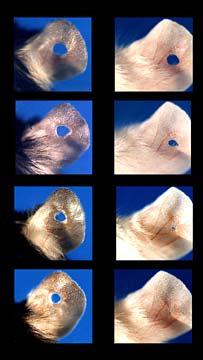
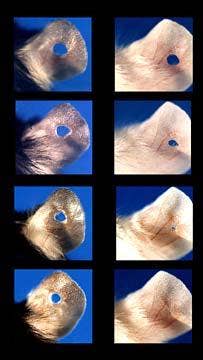
More than twelve years ago, Dr. Ellen Heber-Katz at the Wistar Institute did what so many great scientific minds have done throughout time - she discovered something by accident. In particular, she found that a particular breed of mouse used in lab experiments (the Murphy Roth Large or MRL) could regenerate and heal a hole punched in its ear. Normally reserved for salamanders, newts, and other lower order animals, regeneration in mammals is one of the Holy Grails of medicine. Naturally people were excited by the news. Over the last decade, Heber-Katz and others have shown that the MRL could not only repair a hole punched in an ear, it could repair heart damage as well. Every few years, there's a new break through in this research. 2010 saw the Wistar Institute team demonstrating that the MRL's regenerative traits can be induced in other mice by manipulating the p21 protein. This is some really cool news - regenerative properties in mice by changing just one gene and protein pathway! ...But don't buy into the hype that human regeneration is just around the corner.
Medicine has been taking promising steps forward with healing major wounds. Results with stem cells, transplants, nanotechnology, and tissue printing have all shown the potential for repairing or replacing large sections of our bodies. Actual regeneration, however, is still a very enticing prospect. We'd all like to regrow a missing limb like a lizard. The MRL mice heal tissue damage without need for treatment or special equipment. Furthermore, this ability has been induced in other mice (in a limited fashion) through manipulation of a single gene. That raises some hope that humans could be given a similar ability through a relatively direct treatment (protein regulation or gene therapy).
Part of the recent news really fuels that hope. Heber-Katz and her colleagues are well on their way in discovering exactly how MRL mice are able to regenerate. As described in PNAS, the Wistar team had previously seen that the p21 proteins (related to cell reproduction) was not expressed in MRL ear wounds, so they decided to manipulate that protein pathway in a non-MRL mouse. This non-regenerative mouse had it's p21 protein suppressed (down-regulated) and then demonstrated the characteristic ear-healing of the MRL. Turn off one protein and presto - a new healing mouse.
But we have to avoid the hype. This is certainly very incredible news, but implying that we're actually close to human regeneration ignores many potential problems and limitations. The biggest problem is cancer. p21 (and it's related protein p53) play crucial roles in making sure cells don't reproduce erratically or too often. If we begin to tinker with these protein pathways, we run the risk of defeating your body's failsafe mechanisms that keep cancer in check.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


There's also some clear limitations to the MRL regeneration Heber-Katz and others have observed. Heart damage and ear damage are healed very consistently. Lost limbs are not. The tip of a digit (finger) on the mouse will regrow. Cut off the joint, and the wound has some promising characteristics (like the presence of the right kind of regenerative cells) but it doesn't actually grow back. Not exactly as lizard-like as we would hope.
Finally, there's the biggest reason to not over-hype this latest discovery -it's in mice! Rodent tests are very important, and they are a promising step towards work in humans. We should always keep in mind, however, that even when scientists clearly understand a phenomenon in mice, that there is only promise, not guarantee, that the phenomenon can be brought to people. And it usually takes years for such translations to occur when they do happen.
What then, are we to make of the continued MRL saga? To me, it's all about the genes. Heber-Katz and her crew have understood the importance of the genetic angle from the beginning, seeking from the onset to hunt down the DNA responsible for the ability in MRL mice. At the hub, we've consistently seen that while most important physical traits are a result of a complex interplay between genes, there are the occasional cheats and short cuts. A single gene in rodents helps boost intelligence. Another gene may help increase lifespan in mice by 20%. There are single genes in humans which may be crucial to longevity as well. We also see small gene groups determining a large portion of risk for diseases. Understanding these genetic connections is going to be crucial in the years ahead. Thankfully, our technology for collecting genetic data is poised for impressive gains. Complete Genomics and Illumina are in an arms race to bring us cheap whole genome sequencing. We're going to learn more about the way genes effect our health, whether through regeneration, longevity, or whatever. So, the latest p21 protein discovery about MRL mice regeneration may be just a single step on a long road to human regeneration. Developments in genetics, however, may help us start to run down that path very soon.
[image credits:Wistar Institute]
[source: PNAS, Wistar Institute]
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Google DeepMind AI Decodes the Genome a Million ‘Letters’ at a Time
What we’re reading