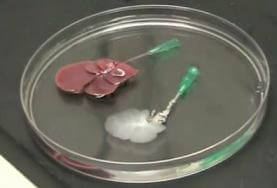
Researchers at Massachusetts General Hospital have successfully grown new livers on the remains of old ones. Using a series of detergents, scientists were able to strip the cells from a rat liver (decellularization), leaving only a collagen scaffold. According to results published in Nature Medicine, healthy liver cells placed on the scaffold developed into a new organ (called recellularization) which was capable of surviving ten days in the lab, and 8 hours inside a living rat. While similar techniques have already been shown to work with other rat organs like the heart, this is likely the first generation of a new liver using the process. If ultimately successful in humans, the scaffolding approach to liver growth could save the lives of thousands of patients in need of a transplant. This is another amazing step forward for regenerative medicine, and the pursuit of being able to replace, regrow, or repair any part of the human body.
Modern transplants require exhaustive matching between donor and recipient in order to avoid lethal immune system responses – the ‘rejection’ of the donated organ. This means that many organs, such as livers, are discarded each year because of lack of compatibility. Even more livers are thrown out because they are damaged or otherwise unfit to be implanted. Around the world, hundreds of thousands of donor livers are destroyed each year because they cannot be used. Yet we have thousands of patients who die each year waiting for a liver transplant. That’s just wrong. And this scaffolding based research is a big step towards making it right. The liver that becomes the scaffold can have damaged or unfit cells – these are stripped away in the decellularization process. Healthy liver donor cells, which are much easier to procure than whole livers and may eventually be able to come from the patient themselves, are added to make the new liver. It’s a solution that could save thousands of lives.
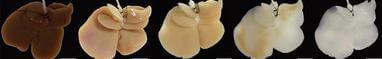
Building a new liver from an old one is a very delicate procedure. Decellularization strips the donor liver of its cells, leaving just proteins and sugars in a complex vascular network. Hepatocytes and endothelial cells are added to this scaffold and allowed to culture. Technology Review has a great interview with the lead researcher for this project, Basak Uygun from MGH, wherein she describes the process in greater detail. You can find that video here.
Recently we discussed a young boy in the UK who had his trachea replaced with a new one created using a collagen scaffold and stem cells. Anthony Atala is growing bladders in his lab. So why is this liver research still working with rats? Not all organs are created equal. The liver requires a steady flow of nutrients and oxygen in order to stay alive, much more than a bladder or trachea. The scaffold provides a wonderful microvascular system that allows for grafted cells to thrive, but it’s not perfect. Clots and other constrictions can develop once the scaffold is transplanted, which is part of the reason why the new livers were only kept in the rats for eight hours.
Despite the slow progress, Uygun and her colleagues are confident that they can perfect a liver scaffold in the near future. According to her comments to Technology Review, Uygun thinks they’ll have a successful rat implantation in the next two years. She expects that human clinical trials could start in five to ten years. That’s fairly optimistic when you’re still in rats and only able to keep a liver alive for eight hours in the body…but I really hope that Dr. Uygun is right.
It’s pretty clear that the creation of organ scaffolds through decellularization, and the subsequent creation of a new working organ through recellularization, is a promising technique to keep an eye on. We’ve already seen it work in vivo with the trachea, and there are many other organs and tissues (like blood vessels) that are simple enough to be tried soon. These human-based scaffolds, however, still require a human donor even if they remove the need for matching. That’s why similar processes using pig or completely synthetic scaffolds (as seen with breast tissue regrowth) may be a better solution in the long term. Eventually we may have the means to grow new healthy donor cells (most likely stem cells) directly on the decayed or damaged organ, with no need for any kind of transplant. At that point, we’ll be approaching a sort of effective immortality, with a steady supply of new cells replacing old ones and keeping us alive. Regenerative medicine is still in its infancy, but once it fully develops it has enormous potential.
[image credits: Technology Review, Uygun et al, Nature Medicine 2010]
[source: Technology Review, Uygun et al, Nature Medicine 2010]


