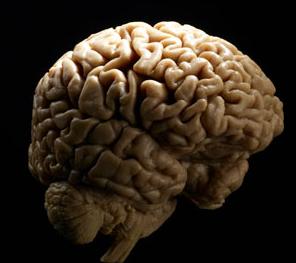
Scotland Injects Stem Cells into Man’s Brain to Heal Stroke Damage
Share
There are few medical calamities that terrify as many people as a stroke. Of those that survive the sudden blocks or ruptures in their brain, nearly half suffer permanent damage that will never be repaired. Researchers in Scotland could be changing that. The University of Glasgow's Institute of Neuroscience and Psychology recently injected fetal stem cells into the brain of a stroke survivor 18 months after his near fatal injury. The man, who is in his 60s, is the first patient in a clinical trial to test the safety and feasibility of using stem cells to repair ischaemic stroke damage (which accounts for 80% of all strokes). According to the University of Glasgow, his injection is pioneering the use of stem cells for this condition, and the purveyor of these cells, ReNeuron, says it is the first UK company to get approval for a human stem cell clinical trial in the country. While it will be months before we are likely to know if the treatment has helped heal the damage in this man's brain, the possibility of success is yet another sign that stem cells are the most promising technology of the early 21st Century.
Look through the hospital beds in the UK and you'll find that nearly one in four of the people in long-term care are there because they suffered a stroke. There are 150,000 stroke victims in the UK and 700,000 in the US each year. Because strokes often leave patients alive but critically impaired they are responsible for billions in healthcare costs. That price tag is only going to increase as the global population continues to age. Finding the right therapy will be critically important in the years ahead.
Stem cells, then, provide a unique opportunity to improve the lives of millions while saving billions, which is the reason this Pilot Investigation of Stem Cells in Stroke (PISCES) was begun. ReNeuron's therapy, ReN001, is derived from the cells of a 12 week old fetus collected in the US (the cells are sometimes designated as CTX). At that phase of development the cells are already differentiating into nerve lineages. It's hoped that the stem cells, injected into the putamen, will release chemicals that stimulate the growth of new neurons and blood vessels. Animal models have already shown how similar injections can reduce inflammation and heal scar tissue associated with ischaemic stroke, as well as promote the growth of new vascular tissue.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


There is a lot riding on this first unnamed male patient. While the University of Glasgow and ReNeuron plan on having 11 more clinical trial participants (all between 60 and 85, and all 6 to 24 months after stroke), they have yet to be completely approved. Safety monitoring agencies in the UK will need to review the first patient's condition in December, and only after that will the others be enrolled and given injections. Varying amounts of CTX will be used. The first patient received around 2 million cells, while subsequent injections in other subjects will increase to 5M, 10M, and 20M. Patients will be monitored for 2 years after injection, with follow up examinations continuing into the long term. Because the fetal stem cells are already differentiated into nerve lineages their risk for producing tumors and cancer is deemed to be less.
This study (which has a registered clinical trial number in the US) is really only aimed at determining safety and feasibility. As such, doctors in Glasgow and representatives at ReNeuron aren't overselling the fetal stem cell therapy at this point. Still, there's a reasonable expectation that patients could heal some level of brain damage, and possibly regain some lost motor skills and functions. The real dream would be for stem cell therapies to completely reverse the changes caused by the stroke. That level of healing is far from expected in this clinical trial, if it is even possible at all.
Like other fetal and embryonic stem cell projects, ReN001 has the potential to heal many people using the same line of cells. The stem cell injections are almost like drug doses. Unlike many other projects that use the patient's own stem cells (autologous transplants), here ReNeuron provides the cells for each patient. We've seen similar work in the US with Geron's spinal cord injury trials, but in general US stem cell research (outside of California) seems to be lagging behind in this field. China, meanwhile, has been going on a trial and error rampage for the last few years, injecting stem cells into every part of the body. Chances are that they've treated a stroke victim at some point with some kind of stem cells, but without the rigorous methodology of this UK study. Hopefully PISCES, which has such an enormous potential to heal patients, will not only lead to success in these UK trials, but encourage similar work to accelerate in the rest of the world. There are a lot of grandchildren out there who'd like to be able to play with their grandparents. Strokes can rob us of those experiences, but stem cells could bring them back.
[image credit:Guardian]
[sources: Guardian, ReNeuron Press Release, University of Glaskow Press Release]
Related Articles

Hugging Face Says AI Models With Reasoning Use 30x More Energy on Average

Study: AI Chatbots Choose Friends Just Like Humans Do

AI Companies Are Betting Billions on AI Scaling Laws. Will Their Wager Pay Off?
What we’re reading