FDA Approves ACT’s Embryonic Stem Cell Trial For Blindness – Should We Get Excited?
Share
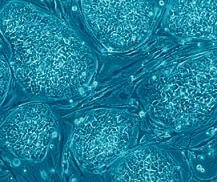
For only the second time in history, the US FDA has granted approval for clinical trials for a therapy derived from human embryonic stem cells. Advanced Cell Technology (ACT), a Massachusetts based bio-firm, recently announced that they had secured approval for human trials of their retinal pigment epithelial cells to treat Stargardt's Macular Dystrophy. SMD causes blindness, generally among youths 10 to 20 years in age, and affects less than 200,000 people in the US. The recently approved trials will only involve 12 patients, and are looking primarily to establish that using the ACT cells is safe. There is hope, however, that the vision of those treated could be improved or restored. In the longer term a success for embryonic stem cells here could lead to treatments for other forms of blindness. Yet as exciting as this study may be it's also a sad reminder of how long it has taken to get embryonic stem cells approved for human trials.
Let's start with the best news first: ACT is on the (relatively) fast track for getting their Stargardt's treatment to market. As we mentioned back in March, they received orphan-drug status for their retinal pigment epithelial cells (known as MA09-hRPE). That status, granted to drugs that have a relatively small and needy prospective patient base, gives them access to better funding, and accelerates the clinical trial process. The effects of this status have already been seen: ACT received NIH funding in June, and $1 million in government grants in November. They also secured patents for their embryonic stem cells in June (though this may only be partially related to their orphan status). Now, ACT is also approved to start Phase I clinical trials, and overall it looks like the FDA is treating ACT fairly well. ACT is even enjoying a small upswing in their stock (OTC:ACTC) with the recent FDA approval announcement.
While Stargardt's is a relatively rare condition, other macular diseases are not. There are millions of people with full or partial blindness related to macular degeneration, with a potential US and EU market measured around $25 to $30 billion (according to ACT). 10% of those aged 66 to 74 are affected by these types of blindness, up to 30% in the 75 to 85 group. ACT may be bursting onto the field through an orphan-drug status, but the greater possibilities extend far beyond the (comparably) small population of those that suffer from SMD.
If ACT can prove the safety of their MA09-hRPE cells during this first phase of clinical trials it looks like they will be on the verge of great things. Yet this potential success is a little tainted in my mind by its scarcity. Besides ACT, the only embryonic stem cell derived technology to get FDA approval for human trials in the US is Geron's treatment for spinal injury. As you may recall, the approval process for that trial was long and winding, with many delays as Geron struggled to dot all its 'i's and cross all its 't's before being given the green light. Even ACT, which I feel like has had a relatively easy time of it, had to clear several FDA hurdles before arriving at this recent approval. The company announced its intent to pursue human trials in November of last year, and made strides in April and July demonstrating that their MA09-hRPE cells didn't cause tumors nor spread themselves uncontrollably through the body.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


In general I support regulatory approval - I certainly don't want to take medications that haven't been thoroughly tested. Yet I'm also frustrated, as I know many of you are as well, that promising stem cell technologies are taking so long to get to patients. Both Geron and ACT had remarkable success in their animal models. Geron got mice walking again after spinal injury and ACT had 100% success with getting rodents to grow new retinal cells after treatments (100 percent!). It's disappointing to think that either a) the FDA doesn't recognize the overwhelming potential of these treatments and isn't willing to help them move along as fast as possible OR b) has been helping them, and this is as fast as it possibly can get.
The silver lining in the FDA's rigorous review of embryonic stem cell technology is that it encourages and highlights the work that scientists at ACT (and Geron) have done to get their approval. ACT's Chief Scientific Officer, Robert Lanza, developed a remarkable assay technique that can detect 1 stem cell among 1 million retinal pigment epithelial cells. For the SMD therapy, Lanza only wants MA09-hRPE cells that are derived from embryonic stem cells, not the stem cells themselves. As only 50,000 to 100,000 MA09-hRPE cells are used in each treatment, the 1 out of 1 million detection is more than powerful enough for these human trials. This assay technique was one of the important tools that convinced the FDA that ACT has cleared its hurdles back in April and June.
Despite my general frustration at the slow pace for embryonic stem cells, the approval for ACT's MA09-hRPE is great news for blind patients everywhere. This is one of several exciting developments for treating blindness with stem cells. Hans Keirstead, the originator of Geron's technology, has had success using embryonic stem cells to treat macular degeneration. Non-embryonic stem cells have a proven decade long history of treating corneal blindness. It's still going to take years for these therapies to reach market, but they are out there. I have a lot of hope that, given enough time, humanity will conquer blindness in all its forms using stem cells and related therapies. That hope is reason enough to wade through all the necessary aggravations of government regulation. I really wish embryonic stem cell technologies would reach us more quickly, but even at a slow steady pace their arrival will be well worth the wait.
[image credit: Nissim Benvenisty via WikiCommons]
[source: ACT Press Release]
Related Articles
Single Injection Transforms the Immune System Into a Cancer-Killing Machine
This Light-Powered AI Chip Is 100x Faster Than a Top Nvidia GPU
This Week’s Awesome Tech Stories From Around the Web (Through December 20)
What we’re reading