This Injectable Brain Implant Can Record and Stimulate Individual Neurons

Share
For those who need them most, brain implants have made inspiring strides in recent years. One implant eases the involuntary tremors associated with Parkinson’s disease. Another allows completely paralyzed patients to manipulate robotic arms. This is amazing stuff—but it’s only a rough draft of the future.
Most implants are still sizable relative to the brain, many are rigid, and all require invasive surgery. A new approach aims to change all that by swapping out flat implants with an injectable electronic mesh.
In a recent paper, published in the journal Nature Nanotechnology, a team of Harvard researchers describe the creation of a flexible nanowire mesh with nanoscale electrodes or transistors placed at each wired junction. The mesh is malleable, "soft as silk," and spacious, allowing it to naturally incorporate into the brain and invite nearby cells to organize and position themselves in close proximity.
The research was led by Harvard chemistry professor, Charles Lieber, and an international team of scientists. Rafael Yuste, director of Columbia University's Neurotechnology Center, told Nature it "left a few of us with our jaws dropping" after a 2014 presentation. (Yust wasn't involved in the research.)
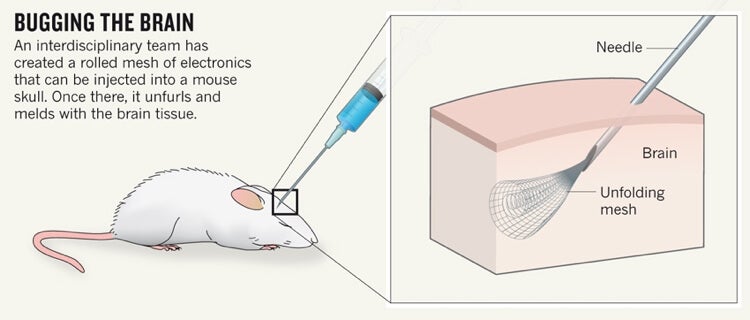
To implant the mesh, a few centimers of the stuff is rolled up in a 100-micrometer diameter syringe and injected through a hole into the brain. This makes the procedure less invasive than current techniques. Once inside, it unfurls and drapes onto the brain’s undulating surface. Nanowires connecting the mesh with computers in the outside world can either record brain activity or stimulate nearby neurons.
The team has tested 16-component implants on mice. They recorded and stimulated individual neurons, and found no indication of an immune response—that is, the body did not reject them—after five weeks.
“Existing techniques are crude relative to the way the brain is wired,” Lieber says. “But with our injectable electronics, it’s as if it’s not there at all. They are one million times more flexible than any state-of-the-art flexible electronics and have sub-cellular feature sizes. They’re what I call ‘neuro-philic’—they actually like to interact with neurons.”
In the future, the team hopes to make the device wireless and scale it up to include hundreds of elements and multiple types of sensors. One such sensor, for example, might be a “hairpin-shaped” nanowire able to measure electrical activity both inside and outside neurons.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


It should be noted, of course, that the research is still in the early stages. Just because it works in mice doesn’t mean it will perform identically in humans. Further, more research and longer trials (beyond five weeks) are needed to establish the tech’s safety. However, though still the bleeding edge, it hints at what’s to come. The potential power of less-invasive, more targeted brain implants and interfaces is significant.
On the one hand, just as brain imaging technology has deepened our understanding of how the brain works, implants measuring neurons in vivo can make that picture even more detailed and complete.
Such research may provide valuable insights into the causes of brain disease and how the brain processes information—opening the door for reverse engineering certain processes in computers, to make them more efficient and, when practical, to allow them to think creatively and make sense of the world more like us.
Also, better brain implants may prove powerful therapeutic tools—whether easing the symptoms of Parkinson’s or restoring a degree of freedom to those suffering paralysis. (And more.) And of course, the less invasive, the better. As the risk profile decreases, the technology will make sense for more patients.
Science fiction writer, Ramez Naam, recently wrote an excellent primer on the current state of the technology and added a glimpse into the far future. Naam calls this the DOS era for brain implants. But as devices shrink, become less invasive and more biocompatible—we're inching toward the iPhone era.
Image Credit: Lieber Research Group, Harvard University
Jason is editorial director at SingularityHub. He researched and wrote about finance and economics before moving on to science and technology. He's curious about pretty much everything, but especially loves learning about and sharing big ideas and advances in artificial intelligence, computing, robotics, biotech, neuroscience, and space.
Related Articles

This Week’s Awesome Tech Stories From Around the Web (Through December 13)

New Immune Treatment May Suppress HIV—No Daily Pills Required

How Scientists Are Growing Computers From Human Brain Cells—and Why They Want to Keep Doing It
What we’re reading