New Technique Heals Wounds With Reprogrammed Skin Cells
Share
People with severe burns, bedsores, or chronic diseases such as diabetes are at risk for developing wounds known as cutaneous ulcers, which can extend through multiple layers of the skin.
Apart from being extremely painful, these wounds can lead to serious, sometimes deadly, infections or amputations. Typically, these ulcers are treated by surgically transplanting existing skin to cover the wound. However, when the ulcer is especially large, it can be challenging to graft enough skin. In such cases, researchers may isolate skin stem cells from a patient, grow them in the laboratory and transplant them back into the patient. But the procedure is time-consuming, risky for the patient, and not necessarily effective.
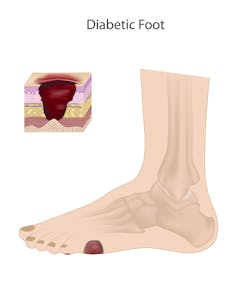
A diabetic foot with a cutaneous ulcer. Such ulcers destroy multiple layers of skin, leading to infections. If untreated, the tissue damage may require that the toes or entire foot be amputated. Image credit: Alila Medical Media/Shutterstock.com
The dramatically rising rates of diabetes alone underscore an urgent need to develop new, effective methods for the treatment of cutaneous ulcers. My laboratory at the Salk Institute focuses on developing stem-cell-based approaches to “reprogram” cells from one type into another for the purpose of regenerative medicine.
In a report in the journal Nature, we describe a new technique to directly convert the cells naturally present in an open wound into new skin cells by reprogramming the wounded cells to a stem-cell-like state, in which cells revert to an earlier, more flexible state from which they can develop into different cell types.
A postdoctoral research associate in my lab, Masakazu Kurita, who has a background in plastic surgery, knew that a critical step in wound healing was the migration of stem-cell-like cells called basal keratinocytes—from nearby, undamaged skin—into wounds.
Basal keratinocytes are precursors to many different types of skin cells. But large, severe wounds such as cutaneous ulcers no longer have any basal keratinocytes. Moreover, as these wounds heal, the cells multiplying in the area, known as mesenchymal cells, are involved primarily in closing the wound and inflammation, but they cannot rebuild healthy skin.
We wanted to convert these mesenchymal cells into basal keratinocytes, without ever taking them out of the body.
To do so, we compared the levels of different proteins inside the two cell types to figure out what distinguished them and find out what we would need to change in order to reprogram one cell type into the other.
We identified 55 proteins, which we call “reprogramming factors,” that are potentially involved in determining and maintaining the cellular identity of basal keratinocytes. We conducted further experiments on each potential reprogramming factor and narrowed the list down to four factors that would transform mesenchymal cells into basal keratinocytes in vitro in petri dishes. These keratinocytes then formed all the cells present in healthy new skin.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


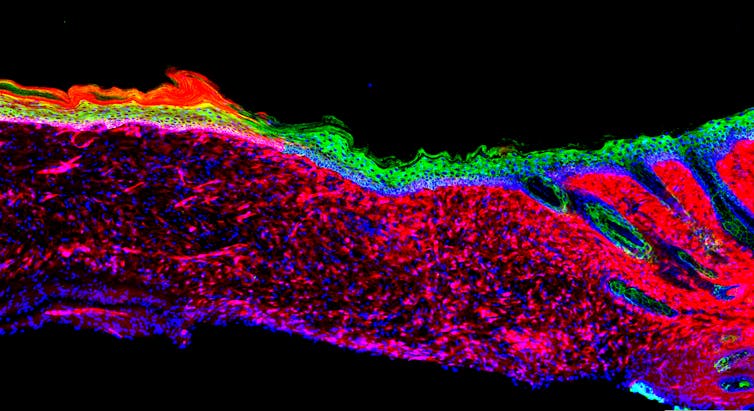
This image is a cross-section of regenerated skin with different cell types indicated with fluorescent tags. Green is keratinocytes, red is mesenchymal cells, blue is the cell nucleus and magenta marks the innermost layer of skin epithelium. Image credit: Juan Carlos Izpisua Belmonte, CC BY-SA
We then tested the power of these four factors to treat skin ulcers on mice. Just 18 days after we applied a topical solution containing these four factors directly onto the ulcers, we saw healing happen. These four factors reprogrammed the mesenchymal cells in the wound into keratinocytes which then grew into the many cells types that make up healthy skin, closing and healing the sore. These cells continued to grow and join the surrounding skin, even in large ulcers. When we examined the mice three months and six months later, we saw that the newly generated cells functioned like healthy skin. Rodent skin heals differently from human skin, so there was no visible scar tissue, though it should have been there.Further work is necessary to ensure the safety of this approach, especially over a much longer term, but as an initial test of the concept, the results are very promising.
We are optimistic that our approach represents an initial proof of principle for in vivo regeneration of an entire three-dimensional tissue, like the skin, not just individual cell types. In addition to wound healing, our approach could be useful for repairing skin damage, countering the effects of aging and helping us to better understand skin cancer.![]()
Juan Carlos Izpisua Belmonte, Professor, Gene Expression Laboratory, Salk Institute for Biological Studies and Adjunct Professor, Cell and Developmental Biology, University of California San Diego
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Image Credit: Kateryna Kon / Shutterstock.com
Related Articles
These Supercharged Immune Cells Completely Eliminated Solid Tumors in Mice

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought
What we’re reading