These Human Mini-Brains Were Implanted in Mice to Pump Out Anti-Stress Hormones
Share
Brain organoids have come a long way. These mini-brains, at most the size of a pea, are made from stem cells or reprogrammed skin cells and churned inside a bioreactor full of nutrients. With different molecular ingredients, scientists can nudge mini-brains to gradually develop striations and structures similar to a growing fetal brain, with the hope of one day replacing faulty brain regions with lab-grown brain blobs.
This week, a team from Japan added a long-overdue superpower to mini-brains: pumping out hormones that control life.
Starting with human stem cells, they grew an unconventional batch of mini-brains that mimic the pituitary gland, the hormone center of the brain. A tiny nugget tucked at the base of the skull, the pituitary is a central highway that links the brain to other parts of the body, controlling stress, metabolism, heart and blood vessel responses, and reproduction.
When transplanted into mice with a damaged pituitary gland, the human cells pumped out a crucial hormone that’s normally secreted by the gland at a steady pace. The transplant lasted for over 24 weeks without any side effects or immune rejection.
The craziest part was that the mini-pituitary was tucked near the kidneys—instead of the brain—underneath a protective fiber-like sheath that enveloped it. Although still requiring surgery, the method shows that when it comes to brain-made hormones that flow into the bloodstream, it’s not always necessary to transplant healthy replacements into the host’s brain itself.
Injecting the mice with a chemical that mimics bacterial infection made the transplanted nugget pump out a hormone from its new kidney habitat to launch a counterattack to battle immune stress.
While far from ready for use in humans with pituitary problems, these “soggy” organoids help bridge the gap between brain and body using their unique form of hormonal ambassadors.
“This method of generating purified pituitary tissue opens new avenues of research for pituitary regenerative medicine,” said study author Dr. Hidetaka Suga of Nagoya University.
The Hormonal Brain
Mini-brains are a breakthrough for neuroscience. They give unprecedented insight into the early stages of human development. They spark with electrical activity. And, perhaps a bit Frankenstein-y, mini-brain chunks can physically hook up to dissected muscle in a lab dish and control their contractions on demand, and restore eyesight to injured rats. Their increasing sophistication even prompted a heated debate on whether these disembodied brainy chunks may become conscious.
Yet they’ve been lacking one major ability: showering the rest of the body with a deluge of hormones. When thinking of brain cells, neurons immediately come to mind. I picture their computational prowess, using electrical signals to weave into dynamic networks that underlie our cognition, learning, reasoning, and memories. But neurons aren’t the only cells in the brain.
There’s a tiny nugget tucked deep inside the back of the head that wields tremendous powers. As a kid, it helps you grow. In adults, it pumps out doses of stress hormones—when needed to keep you on your feet—and maintains a healthy metabolism. The pituitary gland is the grand maestro directing a symphony of hormones that smoothly run your bodily functions.
These hormones don’t come from neurons. Rather, they’re controlled by corticotropic cells in the brain, which constantly consult with their neuronal neighbors on which hormone messenger to manufacture next.
They’re a demanding bunch. These cells are basically the grand central station for sending off hormonal messengers from the brain to the entire bodily landscape. Growth hormones, for example, help stimulate growth and regeneration in the body. Vasopressin commands the kidneys to regulate water and electrolyte retention, important for both battling puffy faces in the morning and maintaining a steady blood pressure. Some hormones drive puberty and fertility. Others, such as oxytocin—also known as the “love hormone”—help a mother bond to her newborn child.
So it’s no surprise that when the pituitary gland fails, the results aren’t pretty. Your blood pressure tanks. You faint without warning. Kids struggle to grow.
The condition, called hypopituitarism, affects roughly 200,000 people in the US. The main hormone that’s lacking is ACTH (adrenocorticotropic hormone), which regulates the body’s stress and immune response. There is no cure. The only way to counteract the disorder is hormone replacement, which can be hard to administer correctly.
ACTH’s levels fluctuate based on a person’s circadian rhythm. Inadequate replacement can ultimately cause a fatal crisis. Too much triggers Cushing’s syndrome, which comes with a myriad of health problems.
So why not have a copy-and-paste brain replacement?
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


A Balancing Act
The team is no stranger to organoids. Back in 2011, they grew 3D tissue from mouse embryonic stem cells that self-organized into mature cells mimicking parts of the pituitary gland. Subsequent studies using human cells also yielded cellular clumps that mimicked the pituitary.
The goal here, the authors explained, was to dial in the recipe for lab-engineered mini-pituitaries ready for transplant.
As a first step, the team nailed down three different molecular signaling pathways that helped closely track how the pituitary develops naturally. Think of it as combining seasonings for a dish to get the perfect mix. They then grew either human stem cells or induced pluripotent stem cells (iPSCs)—chemically reprogrammed from skin cells to resemble stem cells—for over 100 days.
The “recipe” was key. Previous studies added ingredients made from animal products, dubbed “feeders,” which interfered with the growth of these brain blobs. The new recipe, in turn, is feeder-free, yet propelled the stem cells to develop into functional pituitary tissues. The trick was to hijack the brain region’s usual developmental process, adding in two neurochemicals to push their growth along.
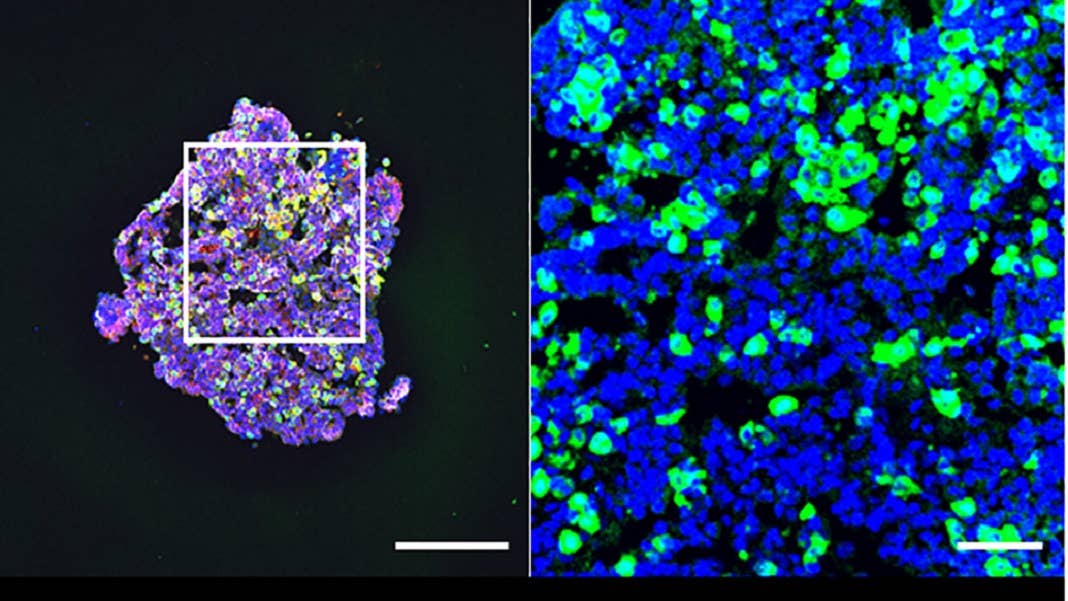
29 days later, the blobs readily secreted ACTH as they floated inside a dish. The hormone levels steadily increased as time went by, and responded to drugs that normally activate the pituitary and its hormonal stress response.
A quick test of the mini-brains’ genetic makeup showed that they have other hormonal tricks up their sleeves. Similar to the real pituitary, the organoids contained cells that, in theory, can manufacture multiple types of hormones.
As an ultimate test, the team transplanted the mini-brains into mice with damaged pituitaries. Eschewing brain surgery, they inserted a 107-day-old organoid right next to the kidneys. The idea sounds bizarre. But here it makes sense: because hormones act on the body through the bloodstream, the pituitary organoid can in theory work even if placed far away from the brain.
The mini-brain graft blossomed next to the kidney. It developed multiple types of hormone-secreting cells with a dose of stem cells, meaning that the transplant could potentially self-renew. Compared to a control group of mice with pituitary damage, those transplanted showed higher levels of ACTH at just four weeks—an improvement that lasted for at least 24 weeks.
When challenged with a myriad of stressors, such as emotional stress or immune stress caused by infections—here, using a chemical mimic—the transplanted organoid revved up its hormone production to combat the assaults.
The mini-brains aren’t perfect. By six months, some already showed decay in their center. But they focused the spotlight on non-neural cells, and are a promising step towards brain organoids that secrete hormones. When further developed, they’re a potential regenerative cure for people who lack pituitary functions—and possibly for other brain-based hormonal disorders.
Next up, the team is planning to further explore stem cells inside the organoids and see if they can maintain the mini-brain’s growth.
“For non-clinical or clinical studies, we will continue to develop manufacturing methods for clinical-grade cell lines, evaluate the efficacy of these lines in animals such as monkeys, and confirm the functionality of products after transplantation,” said Suga.
Image Credit: Arima et. al./Stem Cell Reports
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Google DeepMind AI Decodes the Genome a Million ‘Letters’ at a Time
What we’re reading