Life finds a way.
That’s the conclusion of a new study in Nature, which pitted synthetic bacterial cells against the force of evolution. Stripped down to a skeletal genetic blueprint, the artificial cells started with a losing hand for survival.
Yet they thrived, evolving at a rate nearly 40 percent faster than their non-minimal counterparts. Over 2,000 generations, the streamlined cells regained their evolutionary fitness—the ability to survive, grow, and reproduce—that was initially lost after removing a large portion of their genes.
The results could herald a next generation of synthetic bacteria that pump out insulin and other life-saving medications, produce biofuels, or bio-degrade hazardous chemicals—by tapping into, rather than fighting against, the power of evolution.
The crux was landing on a set of mutated genes that gave the minimal cell an advantage. The same technique might further refine artificial cells by guiding how next generations develop.
Practical uses aside, we can now peek into natural selection itself.
“It appears there’s something about life that’s really robust,” said study author Dr. Jay Lennon at Indiana University Bloomington. “We can simplify it down to just the bare essentials, but that doesn’t stop evolution from going to work.”
Genetic Handcuffs
Evolution is a double-edged sword.
You know the basics. Genes randomly mutate. Most times they don’t have an obvious affect. In some terrible cases mutations kill offspring or cause diseases and haunt later genetic lines.
But rarely, mutations provide the host with a superpower thanks to positive selection, which boosts evolutionary fitness and gives the animal a higher chance of passing down its genes. Examples include squids evolving color-changing skin that hides them from predators or, in humans, skin pigment adaptating to sunshine as we spread across the globe.
Not all genes are equal. Some, dubbed “essential genes,” are critical for survival. These genes mutate but at a very slow rate. Changes are highly dangerous, potentially driving a species toward extinction. Think of these kinds of genes as a house’s foundation—fiddling with them during renovations could cause the whole structure to crumble.
Other genes are far more flexible.
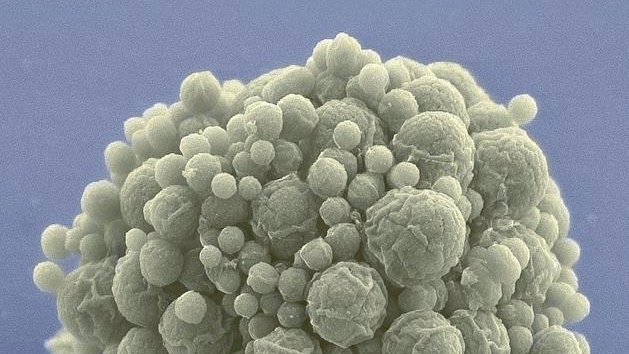
Take Mycoplasma mycoides, a kind of bacteria that often lounges inside the guts of goats. Over millennia, the bugs formed a symbiotic relationship with their hosts, shedding many genes naturally as they increasingly relied on their hosts for nutrition, while keeping genes essential for survival and reproduction. With just 901 genes, M. mycoides is a genetically petite bacteria.
Back in 2016, scientists at the J. Craig Venter Institute further crunched its genome, creating a living creature with just 493 genes. The resulting cell, dubbed JCVI-syn3B, is the simplest autonomous organism to ever grace planet Earth.
Upon learning about JCVI-syn3B at a conference, Lennon was hooked.
“I was blown away by…the analogies of trying to understand something from its simplest basis,” he said. But “if you create an organism that can reproduce, but then you allow it to experience the force of evolution…and mutations and damage that’s going to arise, how does it contend with that?”
The struggle is especially tough for JCVI-syn3B. Because its genome is stripped to the bare minimum, there’s little wiggle room for mutations. When every gene is critical for survival, evolution is Russian roulette—every genetic letter change increases the chances of extinction.
The odds get even bleaker. JCVI-syn3B also lacks protective genes that normally shield cells against mutations, cancer, and death.
We went into the study thinking the organism simply wouldn’t be able to contend with the “inevitable mutations [that are] going to hit one of those essential genes,” said Lennon.
A Minimalist Win
Testing the theory, the team pitted the minimal cell against the first-generation Mycoplasma mycoides (JCV10syn1.0) from which it was derived. Each strain grew in a nutritious broth for roughly 2,000 bacterial generations over 300 days, the equivalent of 40,000 years of human evolution.
It was a brutal trial: based on current estimates, a new mutation could hit every genetic letter more than 250 times during the test.
The first results came as a shock. Although both strains rapidly mutated, the rates didn’t differ. In other words, the little JCVI-syn3B could flexibly modify its genes like its non-minimal cousins, even though the latter had far more genetic letters to tolerate random mutations. Both bacterial strains survived similar types of genetic changes—insertions, deletions, and the switching of genetic letters—without a hitch.
Especially impressive was that the minimal cell came up short for evolutionary fitness at the initial ancestral “weigh in” (that is, before the bacterial cells began their evolutionary journeys).
“The initial effects of genome reduction were quite large; they made the cells sick,” said Lennon. Their fitness—the growth rate or their competitive ability—dropped by 50 percent.
Fast-forward 2,000 generations, and it was a different picture. The minimal cells bounced back, regaining a fitness rate similar to their non-minimal cousins. Despite harboring a bare-boned genome, they readapted to their surroundings and overcame initial genetic shortfalls.
The minimal cells’ main lifeline seemed to be “metabolic innovation.” Rather than adapting themselves to slurp more nutrients from the surrounding broth, the cells instead increased their ability to synthesize molecular pieces of fat into an outer protective layer, without sacrificing the lipid molecules essential for regeneration.
That’s not to say the minimal cells were completely alright. Expanding in size is often a marker of evolutionary fitness—it means a cell can potentially accommodate more proteins and other biomolecules for further growth and division. However, the minimal cell JCVI-syn3B remained roughly the same size, while its non-minimal cousin nearly doubled its heft.
The team has ideas why this might have happened.
Initial tests using CRISPR suggest that one gene in particular may be behind the minimal cell’s petite stature. The cells also lacked half of the usual molecular transporters dotted on their membranes. Like tiny “mouths,” these proteins help a cell catch and absorb nutrients. Fewer molecular mouths turned the cells into picky eaters, which could in turn have harmed their growth.
Another theory suggests cell size doesn’t matter for evolutionary fitness. A cell’s size may just be the fitness byproduct of another genetic trait like, for example, how fast its DNA replicates.
Recent advances in synthetic biology have focused on technological wizardry—such as building genomes for minimal organisms or inserting genetic circuits into bacterial hosts. But answering questions like this is why using synthetic biology to study evolution may be game-changing.
By combining synthetic biology with evolution, we can better understand how genes and their networks function, explained the authors. Ultimately, it may be possible to design and optimize increasingly sophisticated synthetic living systems in sustainable ways.