Scientists Show AI-Generated Proteins Actually Work in Stem Cell Study
Share
Stem cells are finicky creatures.
With the ability to generate any type of cell in the body, they’re constantly bombarded by chemical, hormone, and other signals. Some of these signals nudge them to produce brain cells; others transform them into liver, heart, or kidney cells.
One type of signal, based on a protein called fibroblast growth factor receptor (FGFR), turns them into blood vessel cells throughout the body. FGFR doesn’t just establish our circulatory system. Cancer cells, for example, co-opt the protein to increase their blood flow and survival—at the expense of their host. Scientists keen on regenerative therapies—these replace damaged cells with healthy ones—have been eyeing blood vessels as a key component of tissue repair.
The problem? FGFR signaling is incredibly complex and mysterious.
This week, a new study from David Baker’s lab at the University of Washington, in collaboration with Hannele Ruohola-Baker, used AI to design the perfect trigger for FGFR activity. The duo fashioned a range of AI-generated circular protein molecules to control its signaling.
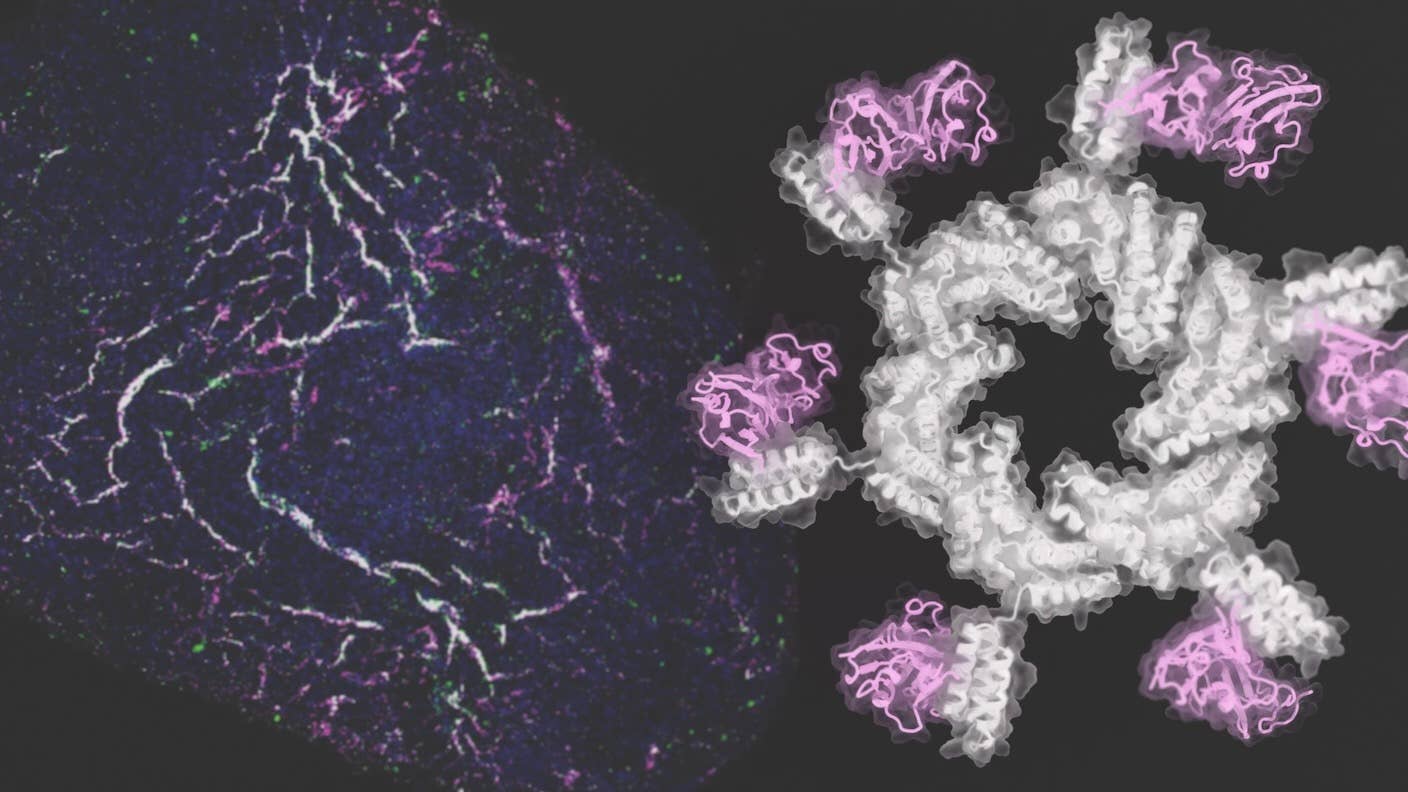
Called oligomers, the structures look like windmills, stars, or butterflies. Tested in human pluripotent stem cells, the team nudged the stem cells into the different types of cells that make up blood vessels.
The oligomers also helped build regenerative tissues. Organoids are a popular way to grow so-called “mini-organs” in a petri dish. One oligomer, combined with another chemical, coaxed stem cells to self-organize into 3D blood vessel organoids. Transplanted into mice, the organoids thrived and connected with the mice’s own blood vessels.
“Whether through heart attack, diabetes, and the natural process of aging, we all accumulate damage in our body’s tissues. One way to repair some of this damage may be to drive the formation of new blood vessels in areas that need healthy blood supply restored,” said Dr. Ruohola-Baker in a press release.
“This collaboration is a case of a biological need propelling a technological advance that could have real-world therapeutic benefit for patients,” she added.
A Cellular Straw
FGFR signaling has been in scientists’ crosshairs for decades. Well-known for its roles in the development of tissues, healing wounds, and cancer growth, tweaking the protein’s activity could shut down blood vessel development in cancers—starving them of nutrients—or speed up recovery after serious physical injuries.
The “R” in FGFR stands for “receptor.” These are the proteins that dot the surface of a cell. Picture a coconut with a straw. The coconut is the cell, and the straw is FGFR. The outside part of the straw interacts with chemicals and proteins and transmits those signals down through the coconut shell—the cell membrane.
The inner part of the straw then relays messages that change the cell’s internal workings, sometimes by tweaking its DNA expression. In stem cells, these signals might urge a cell to rapidly divide and expand or slow down and transform into other cell types.
The protein has another quirk. It usually floats around the cell’s fatty membrane as a single molecule. But to transmit its biological message, individual components need to be physically pulled together, clustering into a brigade. Adding to the complexity is the fact there are four different FGFR genes, which can be translated into two slightly different protein types, called “b” and “c.”
“The pathway is complex and highly regulated,” the team wrote in their paper.
AI to the Rescue
If your eyes are glazing over, you’re not alone. The study aimed to cut through the complexity by designing a protein to reliably nudge stem cells towards functional blood vessels.
They turned to AI. Previous studies have suggested semi-circular protein structures—rather than, say, helixes or sheets—have the most impact on FGFR signaling. The team designed over 100 “arms” to see which could easily dock onto different circular “cores”—these two components make up the final oligomer.
Using RosettaDesign, the team further dialed in their bespoke proteins. Overall, they built an array of oligomers looking like windmills, stars, swirls, or butterflies.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


They also incorporated a a short snippet of amino acids—these are the molecular building blocks of proteins—to help the oligomers grab onto FGFR like Velcro.
Switched On
During early blood vessel development, FGFR drives stem cells to become several different blood vessel cell types. One type protects the vessels like a cushion. Others help the blood vessels maintain their structure. The “c” form of FGFR pushes stem cells towards the first type; the “b” form promotes development into the latter.
The team treated induced pluripotent stem cells cells with a variety of AI-generated oligomers and monitored their growth.
In less than a month, those that activated the “c” form grew an intricate network of blood vessels inside petri dishes. The cells were highly mobile: When the team scratched off some cells, others quickly migrated to repair the gap.
Other types of oligomers pushed the stem cells to form supporting cells that could readily suck up fatty molecules from their environment, suggesting the cells were healthy and worked normally.
Compared to 2D cell culture, 3D blood vessel organoids are far more intricate and difficult to engineer. In another test, the team dosed human stem cells with an AI-generated oligomer. Over 21 days, the stem cells expanded into healthy organoids with complex structures. When transplanted into mice, they incorporated themselves into the critters’ own blood vessels.
The study shows AI-generated oligomers can shift the fate of stem cells—what each cell eventually develops into—at least for blood vessels. It opens a new avenue for regenerative medicine.
“This is a whole new level of control,” said study author Natasha Edman.
Although tailored for FGFR, a similar AI-based approach could be used to control other signaling processes, potentially giving scientists more accurate control of cell growth, maturation, senescence, or death.
“We decided to focus on building blood vessels first, but this same technology should work for many other types of tissues. This opens up a new way of studying tissue development and could lead to a new class of medicines for spinal cord injury and other conditions that have no good treatment options today,” said study author Ashish Phal.
Image Credit: Ian C. Haydon
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Google DeepMind AI Decodes the Genome a Million ‘Letters’ at a Time
What we’re reading