How Tiny Lab-Grown Human Brains Are Giving Big Insights Into Autism

Share
First came lab-grown mini-hearts. Then came 3D printed skin. Now scientists have taken “body on a chip” to a whole new level.
Starting with skin cells from patient biopsies, scientists transformed them back into stem cells, and from those grew pea-sized, self-organizing, crazy-looking nuggets of living — yes, LIVING — brain.
These “cerebral organoids” are the brainchild of Dr. Madeline Lancaster, a neuroscientist at Cambridge who is interested in how our brains develop as embryos. A few years back while working as a postdoc in Vienna at the Institute of Molecular Biotechnology (IMBA), Lancaster noticed off-hand that her cultured brain cells weren’t sticking to the bottom of the dish as usual — instead, they floated up and aggregated into tiny balls.
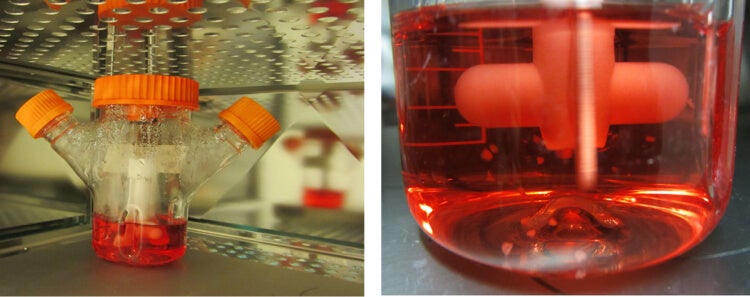
The spinning bioreactor system used for growth of cerebral organoids. On the right, a close-up showing the floating organoids within the bioreactor. [Image/Caption: IMBA/Magdalena Renner]
Curiosity piqued, Lancaster tinkered around with growth conditions until her mini-brains expanded several millimeters wide — tiny compared to a normal human brain, but still something previously unachievable.
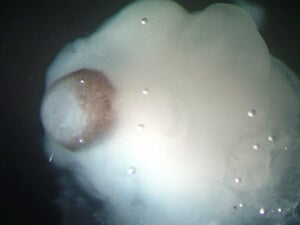
A magnified image of a cerebral organoid showing cerebral tissue adjacent to developing retinal tissue (brown pigmented region). [Image/Caption: IMBA/Madeline A. Lancaster]
The pale, opaque chunks of brain matter didn’t look like much on the surface, but their intricate internal architecture blew Lancaster away.
Under the microscope, the brain blobs were doppelgangers of 9-week-old fetus brains. They contained neural stem cells that busily churned on, making precursor cells that developed into fully functional neurons capable of firing away.
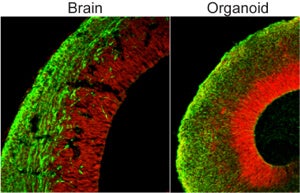
As the blobs grew, the tissue self-organized into distinct subregions populated by different types of neurons — in fact, Lancaster and her team could identify regions in the organoids that roughly looked like a hippocampus, forebrain and even retina. The outer “cortex” of the organoid stratified into rudimentary layers, in a way eerily similar to how our own cerebral cortex matures.
Brain organoids became an instant hit.
Despite their gnarled look, the brain blobs are far more “human” than any animal brain could ever be, making them valuable models for studying human brain development. Plus, unlike actual fetus brains, they can be experimented on.
In a study published last week in Cell, a team from Yale School of Medicine used the technique to glean insight into why autism occurs in some people without a clear genetic cause. The team selected four autistic patients with enlarged heads (a condition that affects roughly one-fifth of people with the disorder), cultured organoids from their skin cells, and compared them to those grown from the patients’ non-autistic fathers.
Within a month, it was apparent that organoids created from people with autism overproduced one type of neuron that acts to dampen the chatter of normal neural activity. This small change upset the delicate excitatory and inhibitory balance in the developing brain, and may in part cause the faulty wiring behind autism’s behavioral symptoms. Further sleuthing led the scientists to a single gene responsible for the glitch.
Before mini-brains, scientists had to shift through gobs of genomic data to fish out gene variants associated with autism. But so far, not a single gene (or even a group of them) can explain the disorder in all its forms and complexities.
The scientists were trying to take the long-winding road from genetics to biology, which proved to be tedious and difficult.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Brain organoids represent a paradigm shift. Scientists can now directly study the biology of complex neurological disorders and from there, nail down the responsible genes. This makes it much easier to home in on potential drug targets for therapy.
Scientists also no longer have to solely rely on mouse models of human disorders.
Although similar, mouse brains don’t exactly follow the same developmental trajectory as human brains, so we don’t know how well the findings translate. And the models are hard to validate: after all, how can one know for sure that a mouse is autistic, depressed or hallucinating?
Then there’s one more perk. Unlike human embryonic stem cells, brain organoids come from skin samples taken from patients with explicit consent. The ethics and legalities are simple.
All this is not to say they’ll be replacing animal models anytime soon.
There’s one major problem: the overall structure of the brain blobs is pretty screwed up. Like facial features on a Picasso portrait, the various subregions are all over the place. They’re also literally dead inside: because the organoids can’t form blood vessels, only their outer layers receive oxygen and nutrients; the core suffocates.
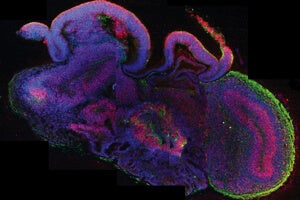
A cross-section of an entire organoid showing development of different brain regions. All cells are in blue, neural stem cells in red, and neurons in green. [Image/Caption: IMBA/Madeline A. Lancaster]
But Lancaster and other organoid-enthusiasts are hard at work. Just last month at the International Society for Stem Cell Research conference in Stockholm, scientists reported updated methods to more reproducibly culture organoids with better architecture. They’re also hoping to coax mini-brains into a later developmental stage by providing external blood supply. With each advance forward, the mini-brain’s complexity and applications grow.
As of now, the organoids most certainly can’t “think”: without external output and mature neural networks to support information processing, their neural activity is nothing more than meaningless cacophony.
In time, however, scientists may be able to grow organoids that resemble a complete human brain. What happens then?
Besides the convenience of a great model, wrote Dr. Arnold Kriegstein, a stem cell researcher at the University of California, San Francisco, in an article accompanying Lancaster’s publication, “there will be a whole new set of ethical and philosophical issues to contemplate.”
Image Credit: Madeline Lancaster/IMBA
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles
New Gene Drive Stops the Spread of Malaria—Without Killing Any Mosquitoes

New Immune Treatment May Suppress HIV—No Daily Pills Required

Scientists Just Developed a Lasting Vaccine to Prevent Deadly Allergic Reactions
What we’re reading