When scientists made immature human egg cells from donated blood, some speculated that human reproduction was on the verge of a massive disruption.
The future is now.
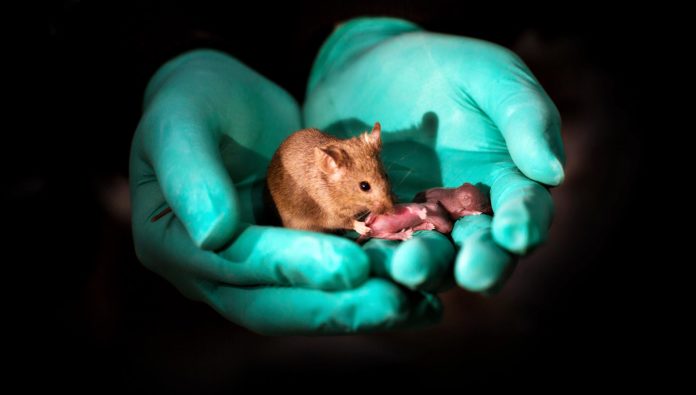
Last week, a team from China completely overhauled natural reproduction, using genetic engineering to make mice born from same-sex parents for the first time. Babies from two moms were indistinguishable from mouse pups generated through good-old-means: not only were they healthy, they went on to give birth to mouse pups of their own.
Those born with the DNA of two dads—with the help of a surrogate—survived through birth but only lived a few days, underscoring the fact that the new technology is still in its infancy.
A masterful technological tour-de-force aside, the study pinpoints critically important genetic factors that allow an embryo to develop normally. It’s like cracking the biological Enigma code of reproduction—a set of genetic instructions that allow an embryo to survive, regardless of how it’s made.
“This shows us what’s possible,” said senior author Dr. Wei Li at the Chinese Academy of Sciences. The team described their results in Cell Stem Cell.
Imprinting Dilemma
How can egg and egg (or sperm and sperm) produce offspring?
The feat may seem less daunting if you think of DNA as computer code: when egg and sperm meet, their biological code (DNA) mix in a way that generates a healthy embryo. “Egg” and “sperm” aren’t that mysterious if they’re simply considered carriers of essential DNA code—23 sets of chromosomes each to be exact, thus allowing the baby to have a normal 46.
But there’s a problem: mammals need DNA code from both the mom and dad to make babies, whereas other parts of the animal kingdom—hammerhead sharks, komodo dragons and other reptiles, fish and amphibians—produce offspring fine without a partner.
Why?
Since the 1980s, scientists have been scratching their heads. Earlier studies first suggested that it’s because of a biological phenomenon called “imprinting.”
Here’s how the theory goes: when egg and sperm meet, the DNA from each parent meet and immediately butt heads. Mom’s DNA, for example, may lead to smaller babies to ease the birthing process, whereas dad’s DNA wants big, strong babies better advantaged to survive once born.
Nature’s solution to the two fighting parties is compromise: some regions of the mom’s DNA are shut off, leaving the dad’s genetic contribution active in the embryo, whereas other parts of active DNA come only from mom. This process, called “imprinting,” happens for about 100 critical genes, many of which are critical for the embryo’s survival and growth.
In a way, the embryo has the final say on which parent’s gene is turned on—those turned off are tagged with a chemical “silencer,” which ensures that one parent’s DNA contribution remains silent, and a progeny only has one active copy of a gene at a time.
Here’s the problem with same-sex parents: most genes are only tagged for silencing in one parent, leaving the other untagged. Combining the same set of tagged (or untagged) genes in a single embryo—as in what happens in mixing DNA from same-sex parents—leads to developmental defects that result in a miscarriage.
Deleting Tags
If tags are the problem, why not erase them?
In this study, the team first turned their focus on the possibility of two moms. They doused embryonic stem cells into a chemical solution that “tricks” the cells into thinking they’re fertilized, and thus start diving. These cells eventually turned into a mutant species called “haploid cells,” in that they contained only half of the normal number of chromosomes (23), much like an egg from a single parent.
Next came removing the tags. In a trial-and-error process, the team gradually hunted down three essential regions in DNA that control imprinting without harming the embryo. Then, using CRISPR-Cas9, the team deleted those critical stretches of DNA.
The deleted regions harbored the imprinting chemical tag, so wiping them out essentially made the oddity cell more “male,” at least in terms of imprinting patterns.
The genetically-engineered cell (a pseudo-sperm) was then injected into the unfertilized egg cell of a different female mouse. The genetic material combined as during normal reproduction, and the embryo was implanted into the womb of a third surrogate mom.
This Frankenstein-ish process incredibly resulted in 29 live mice from 210 embryos made from two moms. Seven of this cohort went on to have baby mice of their own (in the usual way).
The team faced even more hurdles when starting with two male mice. The first steps were almost identical: making haploid cells that contain only half of the usual chromosomes, similar to a sperm.

This time, the team had to hunt down seven regions in the DNA that control imprinting, and painstakingly delete every single one—all without screwing up other regions critical for embryo development.
There’s more: the team then took an egg cell from a female mouse and sucked out its nucleus, the “home” of most of that female donor’s DNA. The egg thus became an empty vessel containing appropriate chemicals that help embryos grow.
The team then injected these modified “sperm” cells—along with sperm cells from another dad—into the empty egg, reconstituting 46 pairs of chromosomes.
Even that wasn’t enough. The team soon realized they needed tissue from the placenta to nourish the weird embryo. So next they took placenta-forming material from another non-viable mouse embryo and used that to cobble together an artificial embryo that was eventually implanted into a surrogate mother.
Yet despite their best effort, the procedure worked a measly two percent of the time—and even those test-tube mice pups died shortly after birth.
“The quick death of the offspring revealed that there were still some unknown reproduction/development barriers to cross in the production of bi-paternal mice,” said study author Dr. Baoyang Hu.
“Successful reproduction from two males is very rare, and can only be found in specific fish under experimental conditions,” he added, noting that there are likely “more barriers than the bi-maternal mice.”
A Crossed Barrier
Although the study only successfully generated a handful of mice from same-sex parents, it nevertheless crosses a biological barrier previously thought impermeable.
It’s a “new way to produce offspring of same-sex mammals,” said Zhou, alluding to previous studies that have tried to achieve the same.
Previous attempts at making babies from two moms resulted in mice pups that developed a slew of health problems, he said. Other attempts at bi-paternal parents required generating an intermediate mother, in that one dad was given the ability to form eggs—a cool scientific victory, but a process that renders humans infertile.
The study here pushes the frontier of reproductive science forward in a way that may one day be clinically relevant.
The key here is “one day”: the procedure’s success rate is far too low to even consider using it in humans. “I think it’s almost impossible that this would be allowed for clinical application,” said Dr. Jacob Hanna at the Weizmann Institute of Science in Israel, who was not involved in the study.
Any technology that pertains to the health of future generations is held up to incredibly high standards, and for good reason. Even “healthy” mice pups born in this study may have underlying issues not obvious until later in life, or that would only show up on cognitively advanced tests—problems that could be passed on to their offspring.
And going from mice to humans is going to be “at least 10 times more difficult,” said Dr. Yi Zhang at Harvard Medical School.
Yet despite these challenges, one thing is clear: the egg-meets-sperm paradigm of human reproduction is crumbling. Now it’s just a matter of when, not if.
Image Credit: Leyun Wang.