 The Herald and other major outlets are reporting that the “UK’s first genetically selected baby has been born.”
The Herald and other major outlets are reporting that the “UK’s first genetically selected baby has been born.”
This sensational claim is misleading since the practice of genetically screening embryos, as was done in this case, has been commonplace for over a decade now. Such screening, called PGD, is almost exclusively used to look for debilitating characteristics in the embryo, such as cystic fibrosis. What is unique about this particular baby is that the embryo was screened not for a true debilitating disease, but rather for a gene that could potentially be harmful later in the child’s life. The gene, called BRCA1, increases the risk of breast cancer in females by as much as seven times.
This genetically screened baby represents a decisive step down the slippery slope of screening embryos not only for genetic defects that are seriously debilitating, but also for genetic traits that are simply risky or undesirable. Future “parents to be” in the UK and elsewhere may use this as a precedent to support the screening of all sorts of other traits. Screening for genes that are completely unrelated to disease, such as height or intelligence, is therefore a step closer to reality. Selection of the sex of an embryo has long been a widespread practice, serving as another precedent for more relaxed limitations on embryo screening.
For those that want to know more about the field of pre-screening embryos a detailed explanation follows:
Genetic pre-screening of embryos before implantation into the uterus is known in the industry as preimplantation genetic diagnosis, or PGD. Two excellent sources of information about PGD are a wikipedia article and the center for preimplantation genetics, both of which were referenced heavily for this report.
PGD can only be performed on embryos in vitro (in a laboratory). That means the test is always performed in conjunction with an in vitro fertilization cycle. The steps are as follows:
1. Fertility drugs are given to the mother to stimulate the development of multiple eggs in her ovaries
2. Eggs are retrieved from the ovaries using an ultrasound guided needle.
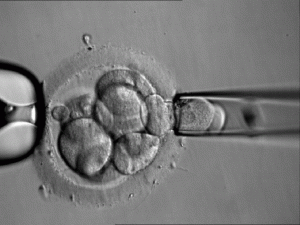
3. Eggs are then mixed with the partner’s sperm in the IVF Laboratory and placed in the incubator for fertilization and embryo growth to the 4 – 12 cell stage.
4. At this point, one or two cells will be biopsied (removed) from the embryo(s)
5. PGD will be performed, which means the biopsied cells will be analyzed for genetic and chromosomal defects/traits.
5. Embryos whose biopsied cells were shown to have an undesirable trait will be terminated. The remaining “good” embryos are candidates that can be implanted into the mother’s uterus for development into a fetus.
Directly from the Center for Preimplantation Genetics:
Misdiagnosis
Misdiagnosis can occur due to mosaicism within the embryo. Some embryos may contain blastomeres (cells produced by the cleavage [division] of a fertilized egg) which are genetically normal and, within the same embryo, other blastomeres which are abnormal. This is called mosaicism. For this reason, a diagnosis may be incorrect. This may result in the transfer of an embryo carrying a chromosome abnormality or the failure to transfer a normal embryo.Experimental error can also account for a misdiagnosis. Improper cell fixation techniques, DNA denaturation errors, allelic drop-out or amplification of contaminated DNA can lead to a wrong diagnosis.
A recent report of the European Society of Human Reproduction and Embryology (ESHRE) documented the PGD results from 25 consortium members from 1999 to 2001. There were 8 confirmed misdiagnoses from 451 PGD tested embryos; 1% (3/305) for chromosome analyses and 3.4% (5/146) for single gene disorders.
Are there risks associated with PGD?
The micromanipulation techniques used for blastomere biopsy are safe with little risk to the embryo. The risk of accidental damage to the embryo during biopsy is less than 1%. There is no risk to the embryo following chromosomal or single gene defect analysis because the analyzed cells are not put back into the embryo. There may be a slightly lower likelihood of implantation after embryo biopsy compared to an embryo not having been biopsied. Other risks may become apparent over time, but to date appear quite limited and need to be weighed against the potential benefits for each couple.
image: cell being removed from embryo (source)


