IBM Research Creates 3D Microscope With 100 Million Times Finer Resolution Than Current MRI

Share
IBM Research issued a major press release today announcing the creation of a microscope that can determine the 3-Dimensional structure of large molecules, bacteria, viruses and other nano sized structures with a resolution of 4 nanometers. This breakthrough, published today in the Proceedings of the National Academy of Sciences (PNAS), represents a powerful addition to the growing arsenal of tools allowing scientists to "see" the 3-Dimensional structure of the nanoworld.
The new microscope uses a technique called magnetic resonance force microscopy (MRFM) to achieve 100 million times the resolution of standard MRI. Using this new device researchers were able to build a 3D image of the tobacco mosaic virus. Although the current results are impressive, the researchers are confident that they can make the microscope a further 10 times more powerful in the coming years, allowing for resolution of 1 nanometer or less.
This microscope from IBM is an incredible scientific achievement that is poised to accelerate the ongoing revolution in the fields of biological research, new medical treatments, and nanotechnology. 3-Dimensional shape is crucial to the proper function of proteins, enzymes, and other molecules in the human body and thus several human diseases result from their malformation. 3-Dimensional shape is equally important in the manufacture and function of nanomachines and nanomaterials where atoms and molecules must be arranged in very specific locations.
Below is a video of the microscope released by IBM, followed by further analysis:
Although this breakthrough is substantial, we should keep in mind that several other techniques (many that also came from IBM in fact) are available for viewing objects at the nanoscale. X-ray Crystallography and cryo electron microscopes have long given us the ability to determine molecular structure, atomic distance, and even "view" individual atoms.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


What is important about this new microscope from IBM is that it will be able to step in where the other techniques have failed. X-ray crystallography requires the structure being analyzed to be crystallized for analysis, a requirement that is not achievable for entire subclasses of proteins and other nano sized structures. Cryo electron microscopy has its own limitations, most notably that the electron beam can destroy the specimen under examination. This new MRFM microscope from IBM will overcome these problems as well as add more flexibility and options to complement the other techniques.
Below are some cool pics with descriptions from IBM media:
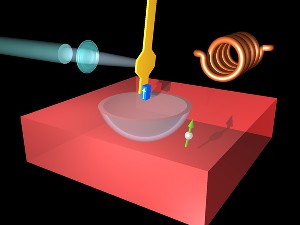 Animation How an MRFM works -- The magnetic resonance force microscope (MRFM) uses an ultrathin silicon cantilever (yellow) with a nanometer size magnetic tip (blue) to detect the magnetic signal from an individual electron buried below the surface of the sample. Because the electron has a quantum mechanical property called "spin," it acts like a tiny bar magnet and can either attract or repel the magnetic tip. The interaction between the spin and the tip is localized to the bowl-shaped region in the sample called the "resonant slice," which moves as the cantilever vibrates. With the aid of a high-frequency magnetic field generated by a coil (right, background), the orientation of the electron (green arrow) flips as the resonant slice passes through. The magnetic force between the electron and magnetic tip alternates between attraction and repulsion every time the electron flips its orientation, causing the cantilever frequency to change slightly. A laser beam (left) is used to measure precisely the variations in cantilever vibration frequency.
|
Related Articles

This ‘Machine Eye’ Could Give Robots Superhuman Reflexes

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

This Brain Pattern Could Signal the Moment Consciousness Slips Away
What we’re reading