Artificial Organelle Unlocking Secrets of the Golgi Apparatus
Share
Don't know how something works? Why not try to build a copy anyway? That's the sort of crazy and amazing thinking that allowed Dr. Richard Linhardt and other researchers at the Rensselaer Polytechnic Institute to create the first artificial organelle. They built a microfluidics chip that allows them to manipulate microscopic droplets of chemicals and mimic the actions of the golgi apparatus. Using the artificial organelle, Dr. Linhardt is working to understand heparin, a chemical used to prevent blood clotting during surgery. The artificial organelle may allow important improvements in the $6 billion worldwide heparin market.
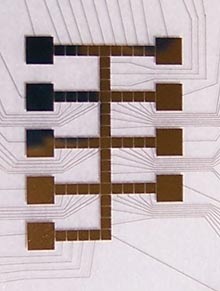
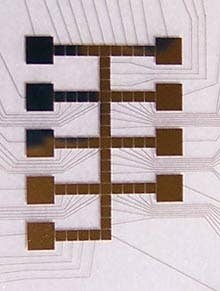
Linhardt, who has studied heparin for close to 30 years, first conceived of using the microfluidics chip to help determine how the golgi apparatus assembles heparin. You see, it's been more than 100 years since the golgi was discovered, but no one really knows how it works. Scientists know that the organelle packages proteins with sugars, they even know which proteins and which sugars. But beyond that...it's the sort of mystery that drives a scientist to quit and start making models.
Which is pretty much what the microfluidics chip is: a working model of the golgi apparatus. Microscopic channels allow very small amounts of different enzymes, sugars, and proteins to be combined, split, and moved. Electrically charged particles and magnets help move things around and give the device some amazing precision. With that kind of control, scientists can model many different reaction times and reactions with hopes of discovering the heparin producing process.

Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Why Heparin?
If you've had major surgery you've probably been dosed with heparin. It's one of the most common anticoagulants and is reliable in its applications. 300,000 doses are used per day in the U.S. alone. For the past 90 years heparin has been made by processing the slimy mucous of pig intestines, which unfortunately leaves it open to contamination. Dozens died last year after receiving bad doses of the drug that originated in China. Linhardt was the one who determined the nature of that contaminant and helped isolate it.
Not satisfied with that, Linhardt went on to create synthetic heparin using fermenting E. coli bacteria. Those bacteria have a similar mucous-y substance as pig intestines. Unfortunately, even brewing bacteria can't make more than a few hundred milligrams of synthetic heparin at a time, about one dose. The world needs hundreds of metric tons. That's where the artificial organelle enters the game.
If Linhardt can better understand how the golgi apparatus makes heparin, he believes that he can improve the amount of synthetic heparin made. Bacteria don't even have a golgi, so finding a way to simulate its processes is pretty important. With the microfluidics cell, Linhardt is learning what he needs to know to improve his bacteria heparin factories. He believes that he is likely 5 years away from producing enough synthetic heparin for clinical trials.
Even understanding the importance of heparin and Linhardt's research, I'm more impressed with the nature of the artificial organelle than I am with its use. Saving lives is wonderful, don't get me wrong, but creating a synthetic model for an organelle may make the more lasting change. We've already seen artificial organs, and a whole range of synthetic limbs; progress on the other end of the scale is much rarer. More artificial organelles could revolutionize the way that we interact with cells. And it all starts with Richard Linhardt and the Mystery of the Golgi Apparatus. Sounds like a Hardy Boys novel.
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Scientists Send Secure Quantum Keys Over 62 Miles of Fiber—Without Trusted Devices
What we’re reading