There have been many proposed means of killing cancer cells: selective pathogens, irradiation, chemical corrosion of membranes…but my favorite has always been “burn the little bastards.” Different researchers have developed means by which nano-sized particles can be attached to cancerous cells and then illuminated with white, UV, or near infrared light. Those nanoparticles then become very heated, burning the cancer they are connected to and leaving healthy cells relatively unharmed. Earlier this year scientists at Argonne National Labs, UC Santa Cruz, and many other centers across the US have made good on large grants from the National Institute for Health (NIH) to develop the next generation of nanomedicine. In preclinical trials, these groups have enjoyed great success in killing brain tumor cells, and melanomas. While it may take years to fully realize the promise of nanomedicine, these results demonstrate that nanotechnology is on the path to defeating cancer.
The NIH’s Roadmap to Nanomedicine outlines how millions of dollars in funding can be awarded to key centers around the country to promote frontiers of nanotechnology used to cure human illness. These awards don’t just focus on curing cancer with nanoparticles, but also include work aiming to create nanosized devices that could repair damage in cells, and molecular machines that could cure chronic conditions (or augment human cells). Over time, this form of dedicated funding from the NIH could cement the US as the leader in nanomedicine.
Brain Tumors Killed by Sunscreen
Scientists at Argonne National Labs and the University of Chicago’s Brain Tumor Center have developed a nanoparticle that can be sent to destroy brain cancer cells. As discussed in Nano Letters, Elena Rozhkova and others on team developed titanium dioxide nanoparticles that could be connected to an antibody that seeks out glioblastoma multiforme (GBM) cancer cells. Titanium dioxide is a fairly common compound found in sunscreens. Once the nanoparticles and antibodies latched onto a group of cultured GBM cells in a lab, the team illuminated the cells with white light for just five minutes. 48 hours later, 80% of those cancer cells were dead. Pretty impressive, though the group has reported that it has some difficulty in getting the nanoparticles to completely avoid healthy cells.
That’s not the only nanomedical techniques that Roshkova and Argonne labs are pursuing. As published in Nature Materials, the team also created magnetic mircodisks that could be used to oscillate near cancer cells. This shaking back and forth damages the cell’s membrane and causes it to die. Using a mild magnetic field activated for just ten minutes, the group was able to kill 90% of cancer cells in a lab setting.
Going for the Gold
If I was going to put my money on one nanoparticle to win in the fight against cancer, I would bet it all on gold nanoshells. These tiny hollow spheres made of gold atoms are only 20 to 70 nanometers across and often only a few nanometer thick. To put that in perspective, most of the light you see has a wavelength ten times longer than these shells’ diameters. These things are small. But they pack a powerful punch. By varying shell thickness and size, they can be tuned to be very sensitive to light in the near infrared (nIR) spectrum. nIR will generally pass through your body without causing any harm, so the shells can be heated inside of your body by light you wouldn’t even see or feel. Unfortunately, most groups have a difficult time producing large quantities of the desired sized gold nanoshells in their labs.
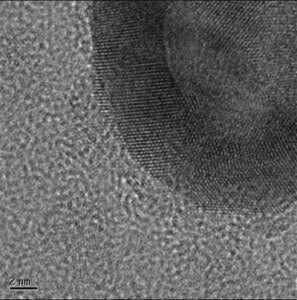
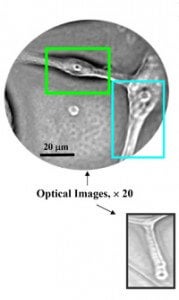
Jin Zhang‘s research group at UC Santa Cruz worked with skin cancer in mice to demonstrate that their gold nanoparticles could be used to burn cancer out of your body. As published in Clinical Cancer Research, Zhang used a special peptide to bind to the nanoshells and guide them to a melanoma. Once there, the team used a nIR beam of light to burn the tumor to death, without significantly damaging healthy cells nearby. The Zhang group was mainly focused on proving the efficiency of their gold nanoshell versus other gold shapes (solid spheres, rods, etc) and it didn’t disappoint. The nanoshell absorbed energy 50% better than other configurations, which is important for future treatments. You want as much energy as possible to be absorbed by each shell so that you can use lower doses of nIR overall.
Gold particles could also be used as detection devices when paired with appropriate biological partner as demonstrated in the following animated video:
Nanoshells are being explored in many different universities around the world. Jennifer West’s group at the Center for Biological and Environmental Nanotechnology at Rice University is also developing means to fight cancer using the nanoparticles. They’ve received their own measure of NIH funding and even had one of their students create the following video to help demonstrate how it might work. You can skip ahead to 3:36 to see the ‘cancer tumor’ burn.
Moving Forward
The use of nanoparticles to burn cancer cells isn’t exactly a new idea. I’ve been hearing about it since I was an undergrad (back in the 1830s) and it’s been a fairly well accepted tactic for the past 8 years or so. In that time there have been some general improvements and common limitations for developing particles into cancer killing machines. First the good news: biologists have a better understanding of what kinds of peptides, proteins, and other biological molecules can be attached to metals to help them be attracted to important strands of DNA, molecules on cell membranes, and other cancer indicators. We’ve seen similar sorts of biological hunters used in other technologies like bacteria sensing, and cancer detecting computer chips. So, we’ve gotten somewhat better at getting nanoparticles to the appropriate place in the body.
Unfortunately, there doesn’t seem to be a general agreement on the ideal size, shape, or composition of those nanoparticles. We’ve already seen how different groups can prefer titanium dioxide, magnetic microdisks, and gold nanoshells. Even small fluctuations in the size of a nanoparticle can have drastic effects on how it behaves in the body. So, each cancer-fighting nanoparticle that gets made is sort of a stand-alone solution for that particular situation.
Which is where the NIH is hopefully going to play a useful role. By spear-heading funding in this important field, the organization can help direct nanomedicine to find the underlying concepts that will guide researchers to find a universal technique for killing cancer. Which is going to lead to some pretty amazing therapies down the line. The National Institute for Health roadmap says that big breakthroughs in nanomedicine should be coming by 2015 (though they’ve wisely avoided mentioning specifics). I look forward to a time when you can go to an oncologist, swallow a pill full of primed nanoparticles, stand in front of a IR lamp and know that you’ll be cancer free in the upcoming year.
[photo credits: Argonne National Labs, Zhang Research Lab at UC Santa Cruz]


