In Landmark Study, Human Stem Cells Restore Monkeys’ Movement After Spinal Cord Injury

Share
Stem cell therapy is highly attractive in its intuitive simplicity: you clean out injured cells, plop down a gang of healthy replacements, sit back, and wait for the body to heal itself.
For spinal cord injuries the potential of stem cells to restore movement seems especially within reach.
But as it happens, the body isn’t quite the simple find-and-replace system. Stem cells when transplanted alone often don’t take, dying off inside the host’s hostile environment before they have a chance to restore function.
For the last three decades, neuroscientists have been scratching their heads, testing cocktail after cocktail of special molecules that can boost stem cell survival. And while there have been successes in rodent models, scaling the therapy to work in primates—a critical step towards human trials—has floundered.
Until now. Last month, a “landmark” study published in Nature Medicine detailed a recipe for transplanted human stem cells to survive and integrate inside the injured spines of monkeys.
Nine months after surgery, the cells extended hundreds of thousands of branches that formed synapses with the monkey’s surviving spinal cord neurons. What’s more, the hosts’ spinal neurons also welcomed the human cells as their own, reaching out to form new connections that restored the animal’s ability to grasp objects.
“The growth we observe from these cells is remarkable—and unlike anything I thought possible even ten years ago,” said lead author Dr. Mark Tuszynski at the University of California, San Diego Translational Neuroscience Institute. “We definitely have more confidence to do this type of treatment in humans.”
The Rodent Trap
Trauma to the spinal cord shears the long, delicate neuronal branches—the axons—that the brain uses to talk to the rest of the body. To restore motor function, scientists need to coax the body to repair or regenerate these connections.
But here’s the problem. After injury the spinal cord quickly reorganizes the extracellular matrix—an intricate web of structural molecules—around the damaged site. Like roadblocks, these proteins effectively inhibit transplanted stem cells from extending out their long-reaching axon branches. What’s more, the injured site also lacks supportive growth factors and other beneficial molecules that act as a nurturing cocoon for the stem cells.
To get around this double whammy, scientists have formulated dozens of growth-promoting cocktails to give the transplanted stem cells a boost. The strategy seems to work.
Back in 2014, Tuszynski transformed skin cells from a healthy human donor, converted them into iPSC cells, and embedded these artificial stem cells into a matrix containing growth factors.
After grafting into rats with two-week-old spinal cord injuries, the human cells fully matured into new neurons, extending axons along the rats’ spinal cord. But shockingly, the team didn’t see any improvement in function, partially due to scarring at the transplant site.
“We are trying to do as much as we possibly can to identify the best way of translating neural stem cell therapies for spinal cord injury to patients,” Tuszynski said at the time.
A New Hope
True to his word, Tuszynski took his transplant protocol to monkeys, which are a much better model for the human spinal cord.
The team cut into a section of the monkey’s spinal cord, and two weeks later—a good approximation for the wait time for patients to stabilize—grafted human stem cells into the injured site along with growth factors.
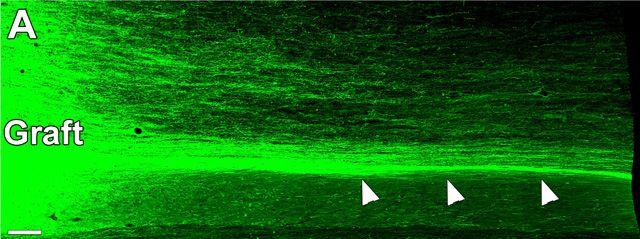
The transplanted human cells were genetically engineered to grow a bright green under fluorescent light. After transplant at the injured site (labeled “graft”), the cells shot out hundreds of thousands of brand new connections (white arrows) within the monkey spinal cord. Image Credit: Mark Tuszynski, UC San Diego School of Medicine
It didn’t work. In the first four monkeys, the grafts didn’t even stay in place.
“Had we attempted human transplantation without prior large animal testing, there would have been substantial risk of clinical trial failure,” Tuszynski said.
The team quickly realized that they needed to boost the amount of a crucial protein ingredient in their recipe to better “glue” the graft in place.
The team also found issues with immunosuppression, timing, and the surgical procedure. For example, they had to tilt the surgical table during the operation to prevent the cerebral spinal fluid—a liquid buffer in the spinal cord—from washing the graft away. In addition, the monkeys required a heftier dose of immunosuppressive drugs to prevent the body from attacking the human cells.
With the tweaks in place, the grafts, each containing roughly 20 million human neural stem cells, stayed in place in the remaining five monkeys.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


The results were stunning. As early as two months after transplant the team found an explosion of new neuronal branches. From the injured site, the stem cells developed into mature neurons, sprouting up to 150,000 axons that snaked along the monkey’s spinal cord.
Some of the branches traveled as far as 50 millimeters from the graft site, roughly the length of two spinal cord fragments in humans. Along the way, they made extensive connections with the monkeys’ undamaged cells.
Even more promising, the monkeys’ own axons also formed synapses with the human neural graft, forming reciprocal connections. These connections are crucial for voluntary arm movements in humans, and this is some of the first solid evidence that transplanted stem cells could form such circuits.
Nine months later, the new neural connections helped the injured monkeys regain some movement in their affected forelimbs, giving them back the ability to grasp soft, squishy objects (for example, an orange) at will. In contrast, the monkeys with failed grafts had little control over fine movements in their palm and fingers—they could only rest the orange on their knuckles.
That may not seem like much, but the authors say that nine months is just a blink in time for functional recovery.
“Grafts, and the new circuitry they were part of, were still maturing at the end of our observations, so it seems possible that recovery might have continued," said study author Dr. Ephron Rosenzweig.
Although the functional improvements were only partial, Dr. Gregoire Courtine at the Swiss Federal Institute of Techonology (EPFL) at Geneva calls the study “a landmark in regeneration medicine.”
"It is not surprising given that the functional integration of new cells and connections into the operation of the nervous system would require time and specific rehabilitation procedures," he said, adding that the study offers valuable insights for potential human use.
Dr. Steve Goldman, a neurologist at the University of Rochester not involved with the work, agrees.
“It’s a big leap to go from rodents to primates,” he said. “This is a really heroic study from that standpoint.”
To Tuszynski, the work is only beginning. For one, not all stem cells are created equal, and his team is trying to determine which ones are most effective at functional repair.
For another, he is also exploring additional ways to further boost the functionality of the regenerated neurons, so that their axons can extend across the injured site and completely replace those lost to injury.
“Patience will be required when moving to humans,” he cautioned, adding that additional safety trials will be necessary before clinical trials. But care pays off.
“There is clearly significant potential here that we hope will benefit humans with spinal cord injury,” he said.
Image Credit: Kamol Jindamanee / Shutterstock.com
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

This Week’s Awesome Tech Stories From Around the Web (Through February 21)

What the Rise of AI Scientists May Mean for Human Research

This ‘Machine Eye’ Could Give Robots Superhuman Reflexes
What we’re reading