How Will Coronavirus End? It Depends on Our Immunity. Three Possible Outcomes
Share
We’re all so ready for this to be over.
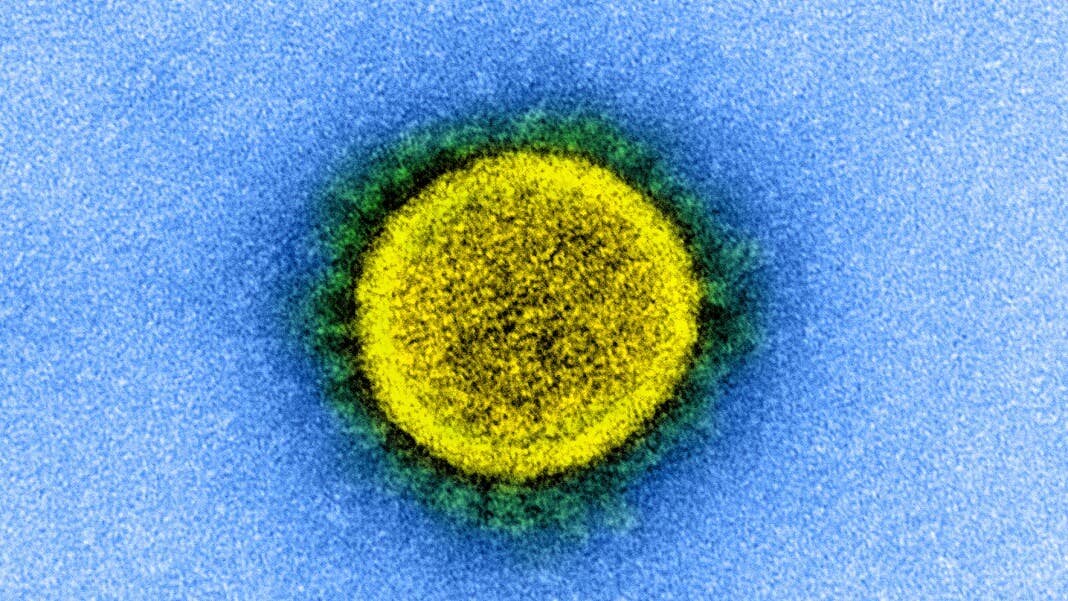
With the curve finally flattening in the US, the ramping up of anti-viral and vaccine trials against SARS-CoV-2—the virus that causes Covid-19—and the launch of antibody tests to screen for previous infection, it seems like science is rapidly moving towards the end game. How exactly the Covid-19 pandemic will finally bugger off into history is still anyone’s guess, but virologists and public health experts generally agree that immunity is key—either through widespread safe and effective vaccination, or when enough of our population has recovered from infections and gained herd immunity.
Well. That’s the hand-waving, shruggie emoji, “eh who knows” short answer.
Like most processes in biology, immunity to SARS-CoV-2 is complex and mysterious, with results that could rapidly diverge into many possible futures. It’s partly why estimates of how long Covid-19 sticks around to wreak havoc can vary enormously, from months to years to…well, seasonal and forever, similar to the flu.
Immunity isn’t just the key factor in determining when we can reopen society. It also underlies answers to personal protection against Covid-19: Could you get re-infected with the same virus? Will vaccines actually work, and for how long? Are positive antibody tests—showing that you’ve previously been infected—actually an immunity passport?
Here’s how immunity works, and a look into possible futures in our war against Covid-19.
Our Badass Immune System
Antibodies may get all the limelight, but they represent just a small fraction of our immune response.
The immune system is basically an entire battalion of rapidly-adaptive units consisting of cellular scouts, killer cell assassins, antibody troops, and intelligence agents that log each encounter with a new enemy. When our body is assaulted by a new foe—viruses, bacteria, or even cancer—surveillance first kicks into high gear.
Normally, a type of peach pit-looking white blood cell called T cells roam around our blood stream. When they detect strange new alien protein bits—often the result of a virus replicating itself—they alert other components of the immune system to organize attacks. These troops have some funky moves: for example, macrophages, the “tanks” of the immune system, can literally swallow up some viruses and digest their genomic material into bits; in some cases, natural killer cells (yup, that’s their name) get pumped up and blast out streams of toxic thermonuclear protein missiles called cytokines that shred the viral invader into oblivion.
But perhaps our best attack comes from B cells, a bulbous, friendly-looking white blood cell that’s also the best weaponry manufacturer in nature. B cells custom-make antibodies—each shaped like a Y with two powerful arms—arranging their sequence in a way that specifically grabs onto a new viral foe. The antibody “hug” is a hug of death; in scientific jargon, it’s called “neutralizing” the infection. Antibodies are extremely specific to a particular virus, which is why testing for SARS-CoV-2 antibodies is generally a sign that you’ve been infected by the virus.
These immune responses aren’t completely benign to the human host. Generally they’ll trigger a fever, and in some very small portion of cases, go berserk in a process called cytokine storm that ends up harming host tissue. These immune overreactions are perhaps why some young Covid-19 patients with no underlying conditions end up dying.
For luckier patients who fight off SARS-CoV-2, the immune system still remains active. Antibodies generally stick around for a bit, though the length depends on the particular virus and could rapidly decline. In a parallel process, the immune system logs the encounter using Memory T cells (also their actual name!), which “remember” specific infections. Memory T cells generally stick around our bodies for a long time after initial infection. If we ever contract the same virus again—“same” being the operative word—these memory cells will rapidly expand into a full-on army to nip the new incursion in the bud.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Three Potential Outcomes
The complexity of our immune response is partly why a positive antibody test may not exactly mean you’re immune to Covid-19, and a negative antibody test doesn’t necessarily mean you’re not immune. Other factors such as the brigades of T cells and the memory for SARS-CoV-2 will also play a role. Given what we know about our immune responses and extrapolating from previous coronavirus (and other viral) infections, there are three likely ways that Covid-19 will eventually end.
Let’s start with the best-case scenario, taken from our response against chicken pox virus called varicella-zoster. Infection with this virus, or vaccination against it and many other childhood infectious diseases, may be uncomfortable, but it’s one-and-done. This means that either direct infection or vaccination can trigger the immune system to remember the virus for a lifetime—to the point that the virus has no chance whatsoever at running rampant again as chicken pox (with the caveat that it can come back as an incredibly painful condition called shingles, but that just shows how complicated our dance with viral attackers is). Generally it means that those who have had the virus and recovered will have immunity for life. If this is how SArS-CoV-2 plays out, as a society we’ll eventually be rid off Covid-19 for perpetuity.
Sound too good to be true? Unfortunately, previous research on coronaviruses suggests that while we’ll have some immunity, it may not necessarily last. In addition, not everyone infected with the Covid-19 virus seems to be able to generate antibodies. However, one preprint study that looked at rhesus monkeys infected with SARS-CoV-2 found that two that were re-infected 28 days after confirmed recovery were able to efficiently fight off the virus. Based on these very preliminary data, it seems we’ll at least have temporarily immunity—that is, once recovered from Covid-19 we won’t immediately get it again. Without human data and time, however, it’s really just an educated guess.
This leads to the second scenario: we get immunity, but it doesn’t last perfectly forever. That is, we could potentially get the virus again, even after initial infection or with a vaccine. The reason is that for some viruses antibodies can taper off after a while, in a sort of “use them or lose them” way. One study on the OG SARS virus that terrorized most of East Asia in 2003, for example, found that antibody levels dropped dramatically after three years from the initial infection. The silver lining in this case, however, is that our immune system retains a memory of SARS-CoV-2, so when memory T cells or leftover antibodies encounter the virus again, they’ll quickly launch an immune response. It’s the difference between a country’s unprepared countermeasures towards an outbreak versus one that’s had previous experience and so has stockpiles of related personal protective equipment and medicines. You might still get sick, but it won’t be as terrible as the first time around.
Finally, the worst-case scenario: the cat-and-mouse seasonal battle. If the virus mutates fast and dramatically enough to outfox our immune system, then our bodies will no longer be able to quickly pick it out from an invader lineup. Our immune intelligence systems and battalions will once again have to fight off a new, if somewhat similar, enemy. It sounds like a frustrating scenario, but it’s exactly what happens with the flu every year. The flu virus mutates at a shockingly rapid rate, which means we’re always one step behind and the virus becomes a seasonal nuisance. The good news is not all viruses have the flu’s superpower. Preliminary studies find that SARS-CoV-2 seems to mutate at a much slower rate than the flu, which is great news for the sticking power of vaccines.
So how does the Covid-19 pandemic end? The uncomfortable truth is no one knows. However, unless SARS-CoV-2 is a total freak of nature, it’ll likely fall into one of the above three categories. They’re not all great. But knowing and planning for the worst-case scenario will make the end more tolerable—after all, uncertainty is perhaps the scariest thing of all.
Image Credit: NIH Image Gallery
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles
In a First, Researchers Use Stem Cells and Surgery to Treat Spina Bifida in the Womb

Hackers Are Automating Cyberattacks With AI. Defenders Are Using It to Fight Back.
This Week’s Awesome Tech Stories From Around the Web (Through March 7)
What we’re reading