Breakthrough CRISPR Gene Therapy Could Be a ‘One and Done’ Injection
Share
CRISPR gene editing has had a big decade. The technology, which earned two of its discovers a Nobel Prize in 2020, can target and edit genes more easily than its predecessors. Still, as tantalizing (and controversial) as the technology's been over the years, it's mostly been developed in the lab.
That's changing now as a growing number of clinical trials are beginning to test gene therapies in humans.
Early CRISPR trials have focused on hereditary blindness and diseases of the blood, including cancer, sickle cell anemia, and beta thalassemia.
Although cutting-edge, the therapies can be costly and intense. In one trial for sickle cell anemia, doctors remove cells from the body, edit them in a dish, and then infuse them back into the patient. In another trial, practitioners inject the gene editing system directly into target tissues in the eye.
Such approaches won't work as readily for other diseases. So researchers and doctors are looking for a general delivery method, like any other medication. Now, a clinical trial from University College London (UCL) has taken a step in that direction.
Participants in the trial suffer from a condition called hereditary transthyretin amyloidosis, in which a mutated gene produces a malformed protein (transthyretin) that builds up in and damages the heart and nerves. The disease is eventually fatal.
Patients received a single infusion of a CRISPR-based therapy into their bloodstream. Blood carried the therapy to the liver, where it switched off the mutated gene and curtailed production of the errant protein. Though the Phase 1 trial was small, the approach had strong results relative to existing options. And it hints at the possibility other genetic diseases may be treated in a similar fashion in the future.
The University of California, Berkeley’s Jennifer Doudna, who shared the Nobel Prize for CRISPR, cofounded Intellia, the company that, alongside fellow biotech company Regeneron, developed the treatment (NTLA-2001) used in the UCL trial.
"This is a major milestone for patients," Doudna said. "While these are early data, they show us that we can overcome one of the biggest challenges with applying CRISPR clinically so far, which is being able to deliver it systemically and get it to the right place.”
A Three-Part Punch
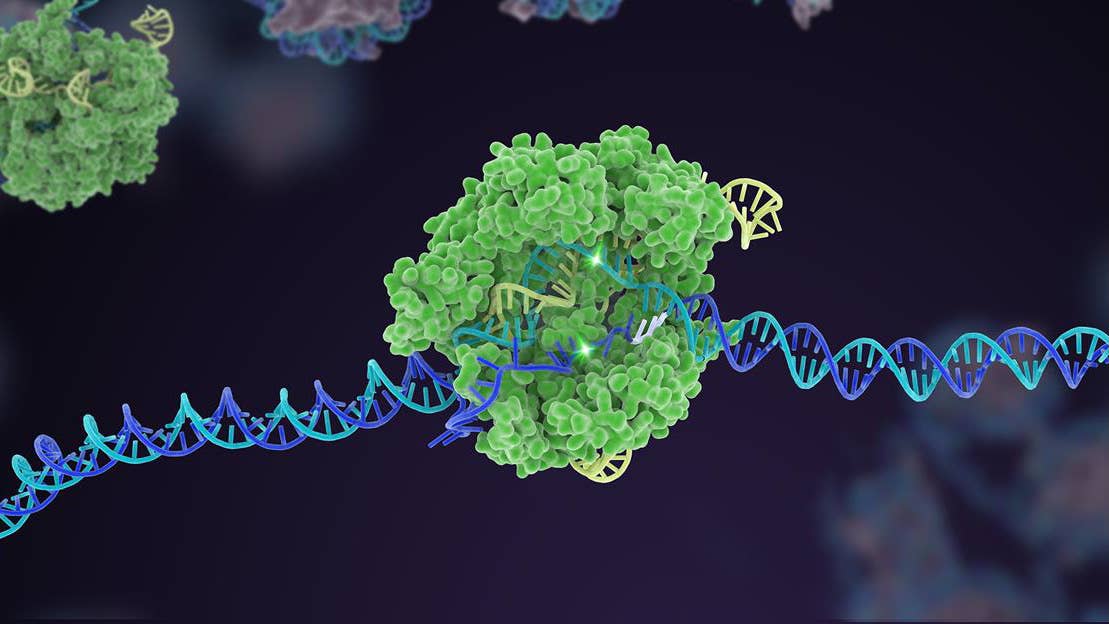
The therapy is made up of three parts. A tiny bubble of fat, called a lipid nanoparticle, carries a payload of CRISPR machinery: a strand of guide RNA and a sequence of mRNA coding for the Cas9 protein.
Billions of these CRISPR-carrying nanoparticles are infused into the bloodstream, making their way to the liver, the source of the dysfunctional protein. The mRNA instructs the cells to produce the Cas9 protein (CRISPR's genetic 'scissors') which then links up with the guide RNA, seeks out the target gene, and snips it.
The cell repairs the DNA at the site of the break, but imperfectly, switching the gene off and shutting down production of the problematic protein.
Interim trial results, reported in the New England Journal of Medicine last weekend, were very encouraging. The trial's six patients, who received either a low or high dose, reported no serious side effects. Meanwhile, production of the target protein declined by up to 96 percent (and an average of 87 percent) in those given the high dose.
One and Done?
The disease, which affects some 50,000 people worldwide, was untreatable until recently.
Existing drugs, approved by the FDA in 2018, silence the mRNA that produces the malformed thyretin protein, instead of altering its gene. They reduce protein production some 80 percent and keep people alive longer, but don't work for everyone and require ongoing treatment.
The CRISPR approach, if successful, would be a one-time treatment. That is, by targeting the genes themselves, the protein is permanently silenced.
Patrick Doherty, a trial participant, told NPR he jumped at the opportunity.
Doherty, an avid trekker and hiker, was diagnosed with transthyretin amyloidosis—which had killed his father—after noticing symptoms, like tingling fingers and toes and breathlessness on walks.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


"It's [a] terrible prognosis," he said. "This is a condition that deteriorates very rapidly. It's just dreadful."
Doherty started feeling better a few weeks after the treatment and said improvements have continued.
"A one-hit wonder," he called it. "A two-hour process, and that's it for the rest of your life."
What's Next?
Although the results are promising, there's reason to temper expectations.
The trial, as noted, was small, and focused on safety. Future work will further test safety and efficacy in larger groups, which—as is apparent from recent experience with covid—can reveal rare side effects or prove disappointing despite early success.
Researchers will likely also look out for off-target "snips" in the liver or other cells. A benefit of this approach, however, is the cells break down the mRNA after they've made the Cas9 protein. In other words, the gene-editing system doesn't persist long.
It also remains to be seen whether the approach would work as well in other diseases. The liver was a prime target for the trial because it greedily soaks up foreign substances. Other organs and tissues may not be as amenable to a general infusion of the therapy.
Finally, the treatment may come with a hefty price tag, perhaps running into the hundreds of thousands of dollars according to Bloomerg's Sam Fazeli.
"Not one person in my field is doing a victory lap, even around their laboratory bench," Fyodor Urnov, a University of California, Berkeley gene editing expert, told US Today. "We're all slightly blue because we're all holding our breath."
If the trial does prove successful, however, researchers will want to know if they can reach any organ or target tissue with a general infusion. And can genes also be edited in vivo? Instead of merely knocking out a faulty gene, can we safely correct it?
In the future, when the kinks have been worked out and the science more thoroughly proven, Urnov said, gene editing could help millions of people around the world with genetic conditions. And this trial, it seems, is a notable step in that direction.
Image Credit: NIH
Jason is editorial director at SingularityHub. He researched and wrote about finance and economics before moving on to science and technology. He's curious about pretty much everything, but especially loves learning about and sharing big ideas and advances in artificial intelligence, computing, robotics, biotech, neuroscience, and space.
Related Articles
Single Injection Transforms the Immune System Into a Cancer-Killing Machine
New Gene Drive Stops the Spread of Malaria—Without Killing Any Mosquitoes

New Immune Treatment May Suppress HIV—No Daily Pills Required
What we’re reading
