Your Brain on Mushrooms: Study Reveals What Psilocybin Does to the Brain—and for How Long
Share
Magic mushrooms have recently had a reputation revamp. Often considered a hippie drug, their main active component, psilocybin, is being tested in a variety of clinical trials as a therapy for the likes of depression and post-traumatic stress, bipolar, and eating disorders.
Psilocybin joins ketamine, LSD (commonly known as acid), and MDMA (often called ecstasy or molly) as part of the psychedelic therapy renaissance. But the field has had some ups and downs.
In 2019, the FDA approved a type of ketamine for severe depression that was resistant to other therapies. Then in early June, the agency rejected MDMA therapy for post-traumatic stress disorder, although it has been approved for limited use in Australia. Meanwhile, healthcare practitioners in Oregon are already using psilocybin, in combination with counseling, to treat depression, although the drug hasn’t yet been federally approved.
Despite its potential, no one knows how psilocybin works in the brain, especially over longer durations.
Now, a team from Washington University School of Medicine has comprehensively documented brain-wide changes before, during, and after a single dose of psilocybin over a period of weeks. As a control, the volunteers also took Ritalin, a stimulant, at a different time to mimic parts of the psilocybin high.
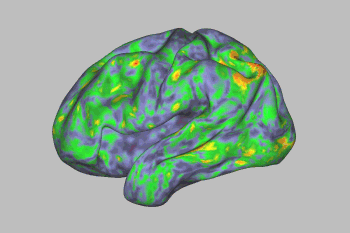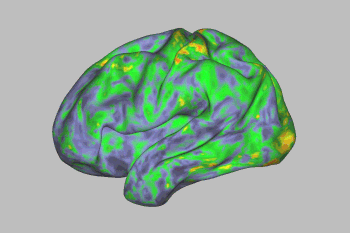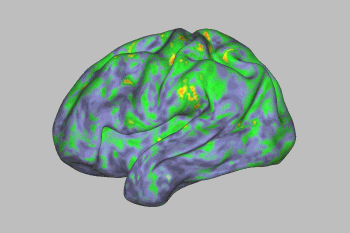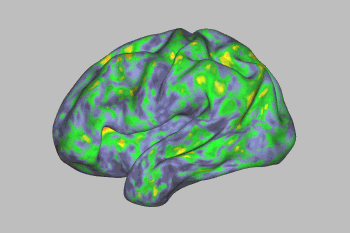
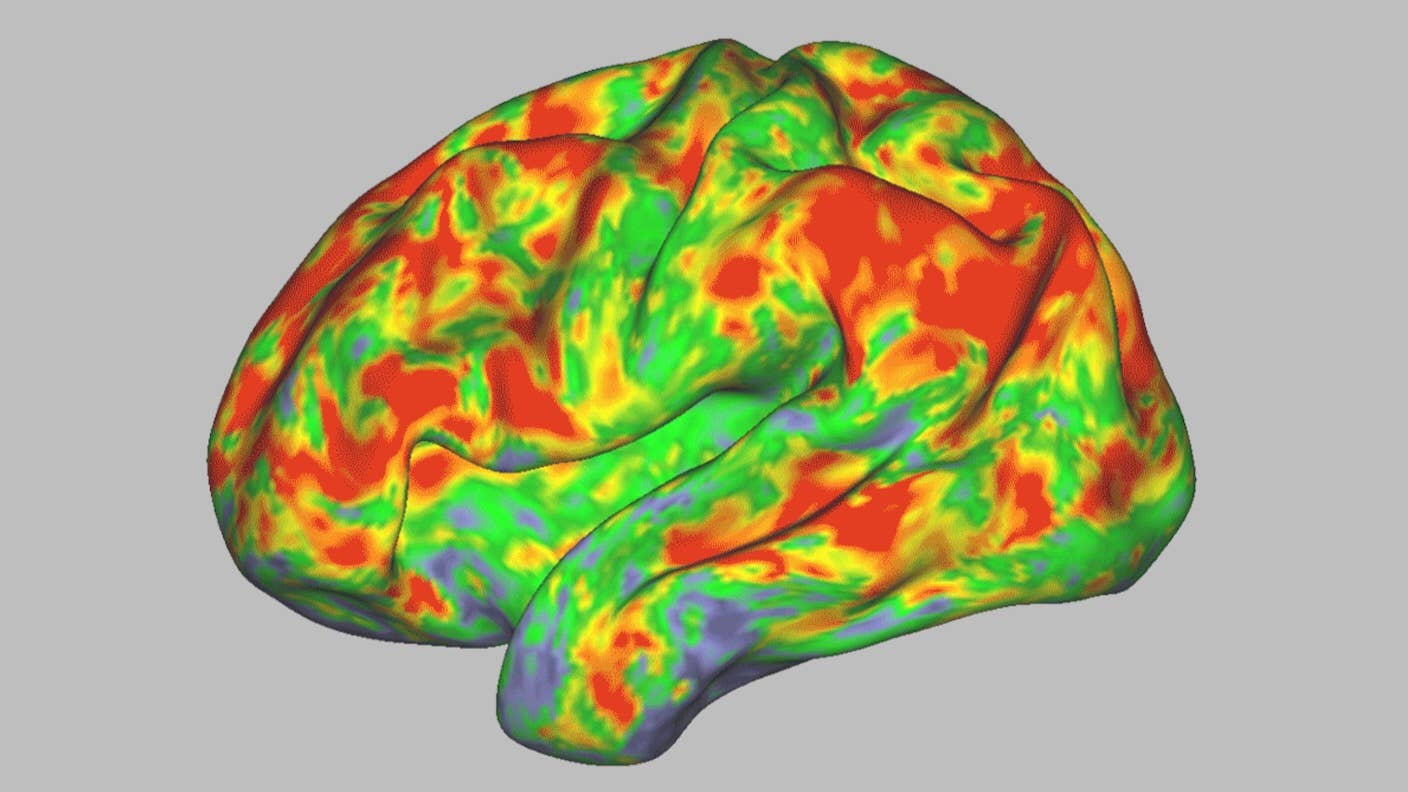
An fMRI scan shows the effect of psilocybin on the brain. Yellows, oranges, and reds indicate an increasingly large departure from normal activity. Image Credit: Sara Moser/Washington University
In the study, psilocybin dramatically reset brain networks that hum along during active rest—say, while daydreaming or spacing out. These networks control our sense of self, time, and space. Although most effects were temporary, one connection showed changes for weeks.
In some participants, the alterations were so drastic that their brain connections resembled those of completely different people.
Normally, the brain synchronizes activity across regions. Psilocybin disrupts these connections, in turn making the brain more malleable and ready to form new networks.
This could be how magic mushrooms “contribute to persistent changes…in brain regions that are responsible for controlling a person’s sense of self, emotion, and life-narrative,” wrote Petros Petridis at the NYU Langone Center for Psychedelic Medicine, who was not involved in the study.
Magical Mystery Tour
The brain’s 100 billion neurons and trillions of connections are highly organized into local and brain-wide networks.
Local networks tackle immediate tasks such as processing vision, sound, or motor functions. Brain-wide networks integrate information from local networks to coordinate more complex tasks, such as decision-making, reasoning, or self-reflection.
Previous psilocybin studies mainly focused on local networks. In rodents, for example, the drug regrew neural connections that often wither away in people with severe depression. Scientists have also pinpointed a receptor—which psilocybin grabs onto—that triggers this growth.
But psilocybin’s effects on the whole human brain remained a mystery.
Several years back, one team sought an answer by giving people with severe depression a dose of psilocybin. Using functional MRI (fMRI), a type of imaging that captures brain activity based on changes in blood flow, they found the chemical desynchronized neural networks across the entire brain, essentially “rebooting” them out of a depressive state.
Daydream Believer
The new study used fMRI to track brain activity in seven adults without mental health struggles before, during, and for three weeks after they took psilocybin. The researchers gave participants a single dose on par with that commonly used in clinical trials for depression.
During the scans, the participants had two tasks. One sounds easy: They kept still and focused their gaze on white crosshairs on a computer screen, but remained otherwise relatively relaxed. Even so, tripping on mushrooms inside a noisy, claustrophobic machine is hardly relaxing—heart rate skyrockets, nerves are on high alert, and anxiety rapidly builds. To control for these side effects, the participants also took Ritalin—a stimulant commonly used to manage attention deficit hyperactivity disorder—at another point in time during the study.
The other task required more brain power. Like an audio version of a CAPTCHA, the researchers asked volunteers to match an image and a word prompt—for example, they’d have to pick a photo of a beach after hearing the word “beach.”
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Throughout the study, each person had their brains scanned roughly every other day, on average totaling 18 scans.
Mapping brain connections over time in the same person can “minimize the effects of individual differences in brain networks organization,” wrote Petridis.
The study found psilocybin immediately desynchronized a brain-wide network, generating a brain activation “fingerprint” of sorts that differentiates it from a sober brain.
Dubbed the default mode network, this neural system is active when the mind is alert but wanders, like when reliving previous memories or imagining future scenarios. The network is distributed across the brain and is often studied for its role in consciousness and a sense of self. The chemical also desynchronized local networks across the cortex, the outermost layer of the brain that supports perception, reasoning, and decision-making.
However, the chemical partially lost its magic when the volunteers were focused on the image-audio task, at which point the scans showed less disruption to the default mode network.
This has implications for psilocybin-assisted treatment. Clinical studies have shown that during psychedelic therapy, a challenging experience—a bad trip—can be overcome by a method called “grounding,” which reconnects the person to the outside world.
These results could explain why adding eye masks and ear plugs can enhance the therapeutic experience by blocking outside stimulation, while grounding pulls one out of a bad trip.
Psilocybin’s effects lingered for a few days, after which most brain networks returned to normal—with one exception. A link between the default mode network and a part of the brain involved in creating memories, emotions, and a sense of time, space, and self was disrupted for weeks.
In a way, psilocybin opens a window during which neural connections become more malleable and easier to rewire. People with depression or post-traumatic stress disorder often have a rigid and maladaptive thought pattern that’s hard to shake off. With therapy, psilocybin allows the brain to reorganize those networks, potentially helping people with depression to escape negative ruminations or for people suffering from addiction to consider a new perspective on their relationship to substances.
“In other words, psilocybin could open the door to change, allowing the therapist to lead the patient through,” wrote Petridis.
Although the study offered a higher resolution image of the brain on mushrooms over a longer timeframe than ever before, it only captured scans of seven people. As the participants did not have mental health issues, their responses to psilocybin may differ from those most likely to benefit therapeutically.
Ultimately, larger studies in diverse patient populations—as in several recent MDMA trials—could offer more insights into the efficacy of psilocybin therapy. For example, the one persistent brain network disruption could be an indicator of treatment efficacy. Investigating whether other psychedelics alter the same neural connection is a worthy next step, wrote Petridis.
With the field of psychedelic therapy projected to reach over $10 billion by 2027, understanding how the drug affects the brain could bring new medications with fewer side effects.
Image Credit: Sara Moser/Washington University
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

This Brain Pattern Could Signal the Moment Consciousness Slips Away
Vast ‘Blobs’ of Rock Have Stabilized Earth’s Magnetic Field for Hundreds of Millions of Years

Your Genes Determine How Long You’ll Live Far More Than Previously Thought
What we’re reading