‘Startling Advance’ in Designer Proteins Opens a World of Possibility for Biotech
Share
Proteins are a bit like lights in your house. They have a job to do, and getting them to do it involves switching them on and off with other proteins or molecules.
But it’s much easier to flip the switch on a light. In the body, billions of years of evolution have generated a complex web of molecular signals that act as biological switches for proteins.
This week, a team led by Dr. David Baker at the University of Washington offered a shortcut.
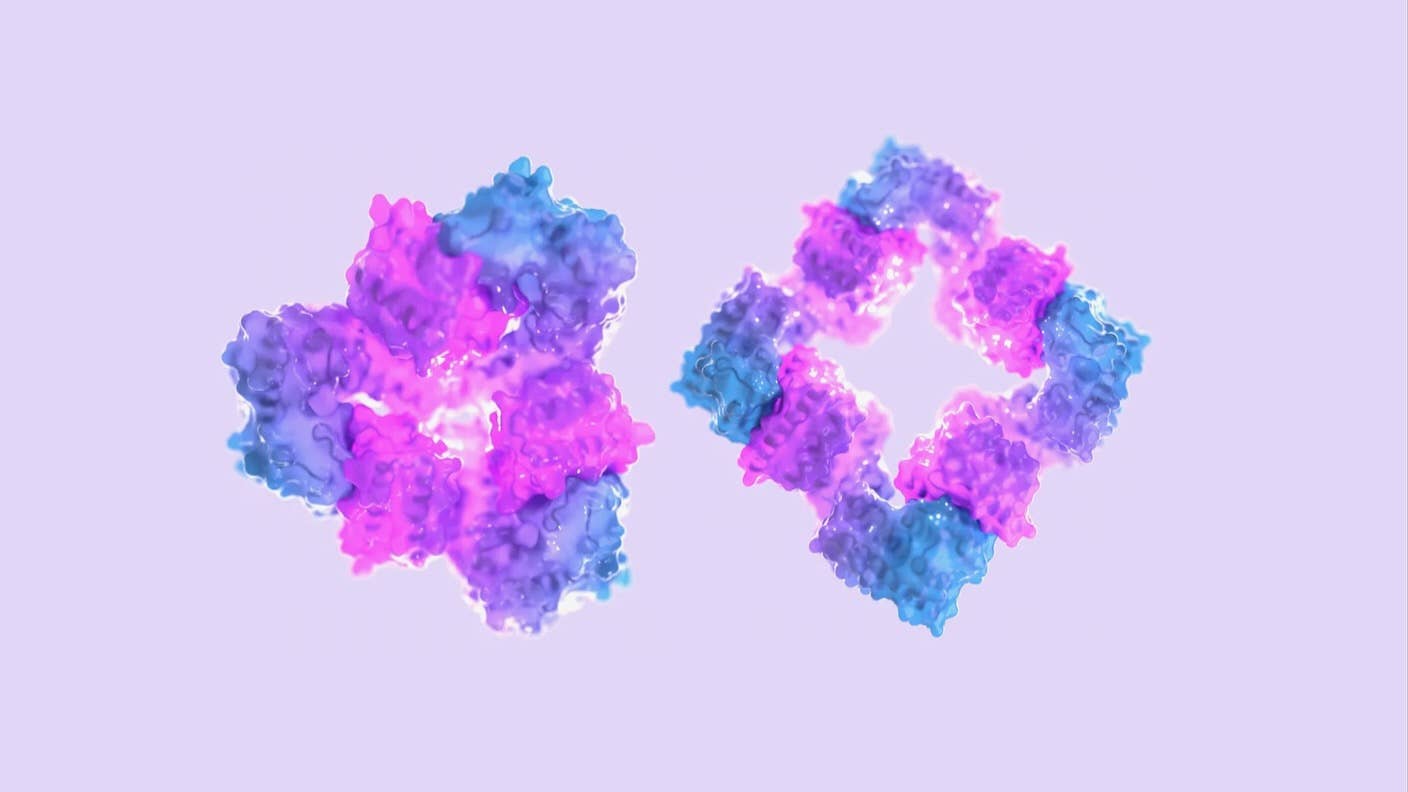
Using AI, they designed proteins that reliably transform themselves in the presence of a molecular switch—dubbed an “effector.” These designer proteins, unknown in nature, contain hinges that allow them to bend and assemble into different structures when dosed with an effector, and then disassemble into individual components when the effector disappears.
It’s a “startling advance for the field,” wrote Dr. A. Joshua Wand at Texas A&M University, who was not involved in the work.
The team designed proteins that can morph into myriad dynamic arrangements, such as rings or cages, loosely mimicking the behavior of their biological peers—for example, how the blood protein hemoglobin assembles to carry oxygen.
Switchable proteins open a world of possibility. Cage-like proteins could carry medication through the body and then, with a molecular flick of the switch, open to release it, allowing triggerable drug delivery. Other designs could potentially monitor disease-causing molecules in the body or pollutants in the environment. In synthetic biology, they could form the basis of biological circuits, acting as tunable switches that can predictably change a cell’s behavior.
“By designing proteins that can assemble and disassemble on command, we pave the way for future biotechnologies that may rival even nature’s sophistication,” said Baker in a press release.
Proteins, Assemble
Proteins are the body’s workhorses. They build and run our bodies. Protein networks determine when cells divide, thrive, or die. Scientists have long relied on proteins to develop vaccines, cancer therapies, and treatments for brain and heart disorders.
Structure is a crucial attribute, especially for larger proteins made up of multiple components. They need a stable shape so they can grasp other proteins and trigger biological responses, but the shape must also be able to change depending on the cell’s needs.
It’s a bit like having planks of wood for multiple house-restoration projects. The planks can combine to make a table, a set of stairs, or a planter for the garden. Similarly, our cells assemble protein “planks” into a variety of shapes—but with a twist.
Take hemoglobin, a protein in the blood that carries oxygen. It’s made up of four protein planks, each able to grab onto oxygen. But they act as a team: When one plank latches onto oxygen, it’s easier for others to do the same.
This type of molecular collaboration has inspired scientists for nearly a century. Here, oxygen is the effector. It flips a protein switch, helping proteins better carry oxygen through the body. In other words, it may be possible to optimize protein functions with an alternative effector drug.
The problem? The original inspiration is wonky. Sometimes hemoglobin proteins carry oxygen. Other times they don’t. In 1965, a French and American collaboration found out why. Each protein alternates between two three-dimensional shapes—one that carries oxygen and another that doesn’t. The shapes can’t coexist in the assembled protein to carry oxygen: It’s all-or-none, depending on the presence and amount of the effector.
The new study built on these lessons to guide their AI-designed proteins.
Shape Shifters
The team tapped several advances in recent years—most of which they’ve led.
One is the use of AI to predict protein structure. Another is the design of a hinge-like protein that changes its shape to take on two different forms (a bit like a biological transistor). The last is an AI that can stitch protein "planks" together into structures.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


The team first used AI to design a group of flexible proteins, each with a hinge and two ridged arms. This setup keeps the protein’s structure stable, but lets it bend at the hinges. The hinge does double duty: It’s also a sensor. In the presence of an effector molecule, the protein changes its shape from a flat plank to a hinged “V” shape.
As a proof of concept, the team synthesized multiple AI-generated proteins and tested them in the lab. In one of these, the proteins formed a ring-like structure when given a customized effector made of peptides, or small protein chunks.
In another test, they designed a protein that grabbed onto another similarly shaped protein in the presence of an effector. Processes like this are often used by cells to change their inner workings, and in synthetic biology, they’re switches that trigger a molecular response—for example, turning genes on or off or altering the fate of a cell. Nearly 40 percent of these designer proteins dissolve in water, making them more compatible with our bodies.
Going further, the team designed a protein with two hinges connected by a short loop. In the presence of an effector, the proteins twisted in a way that mimicked hemoglobin.
Finally, they explored ways to disassemble the proteins.
“This addresses a major current protein design challenge,” wrote the authors.
A useful tool might form a cage that carries and releases a payload of medicine when encountering specific signals in the body. Choosing from the proteins in their repertoire, the team engineered a different effector that broke the cage back down into its components.
Similarly to how proteins assemble in our bodies, the engineered proteins also had the “amp-up” effect, in that grabbing onto an effector made it easier for other components to do the same—in a virtuous cycle. However, the proteins developed in the study are all unknown to nature, opening a new space “unexplored by natural evolution,” wrote the team.
They could be adapted into controllable nanomaterials or drug packaging systems that unleash cargo with a trigger. Other uses include biosensing, which can make cell therapies—such as those for cancer—more traceable, and protein nanobots that morph into different structures.
Still, many challenges remain.
This type of regulation “in nature is much more varied and complicated,” wrote Wand. Whether AI-designed proteins can fully capture the shape-shifting capabilities of natural proteins remains to be seen.
Image Credit: Baker Lab
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought

Google DeepMind AI Decodes the Genome a Million ‘Letters’ at a Time
What we’re reading