A New Gene Therapy Reprograms Cancer Cells to Fight Themselves
Share
Cancer cells are tricky foes.
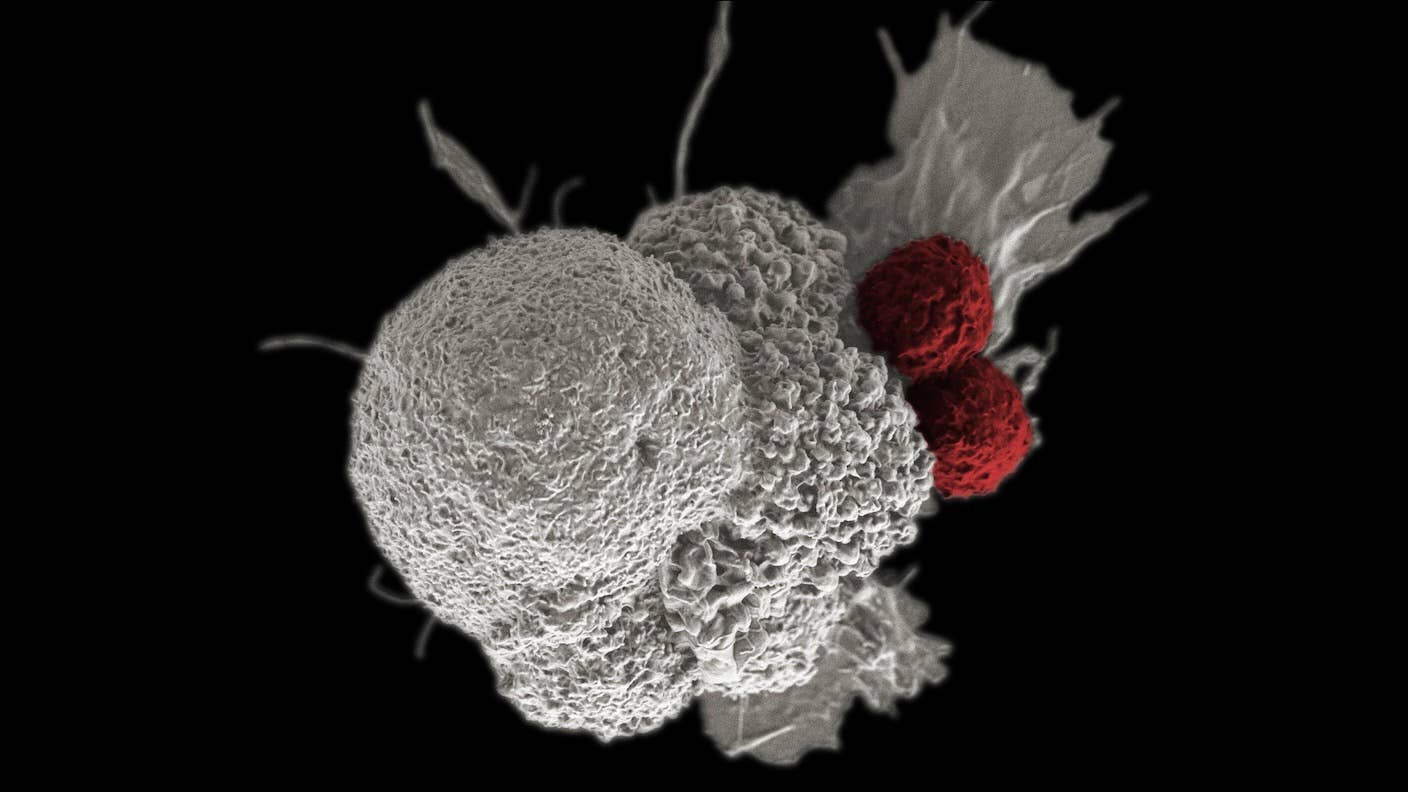
Our body’s immune system is normally on the lookout for signs of tumor cells. If any are detected, it launches killer T cells—a type of immune cell—to seek and destroy the threat. But it’s a cat-and-mouse game: As tumors grow, they form a protective barrier to counteract immune attacks. Immune cells lose their targeting and killing efficacy inside the protective zone.
One workaround is to genetically engineer more powerful T cells. A relatively new and promising approach called CAR T therapy adds more “targeting beacons” onto T cells extracted from each patient to convert them into tailored cancer torpedoes.
So far, six CAR T therapies have been approved by the FDA for various blood cancers. But they have an Achilles heel. Once inside the body, their numbers slowly dwindle, and they gradually lose their cancer-battling abilities.
Some scientists are working to make CAR T cells more deadly. Others are turning T cells into Trojan horses to infiltrate tumors. One such therapy was approved in May 2024, marking the first cellular therapy for a solid tumor—melanoma, an aggressive skin cancer.
Even with these upgrades and alternatives, a tumor’s protective shield is still difficult to penetrate. This month, a team from Asgard Therapeutics and Lund University took a clever new approach to tackle tumors from within. The work was
Using a technology called cellular reprogramming, the team transformed tumor cells in mice into a type of immune cell called cDC1 cells. These cells are master regulators of the immune system. They’re rare inside tumors but when present can trigger powerful immune responses that eat away at the cancer’s protective shield and recruit T cells to the target.
Mice treated with the gene therapy remained cancer-free for at least 100 days and resisted cancer resurgence in a lab test.
“The data provides preclinical proof-of-concept for an off-the-shelf, yet tumor-specific, first-in-class cancer immunotherapy,” wrote Asgard Therapeutics in a press release.
Identity Change
At the heart of the therapy is a technology called cellular reprogramming. Here, scientists use a combination of proteins called transcription factors to turn genes on or off. This process can change a cell’s identity.
The most famous example of cellular reprogramming is the Nobel Prize-winning creation of pluripotent cells (iPSCs). These cells have revolutionized regenerative medicine and how we study diseases. Here, four transcription factors convert mature skin cells back into stem cells. This type of cell can develop into any other type of cell in the body. Additional factors can then gently coax the newly minted iPSCs to assume new identities—for example, brain organoids (“mini-brains”), egg and sperm precursor cells, or liver and bone cells.
Soon after its introduction, the groundbreaking technology showed promise for gene therapy.
In 2008, a study found that delivering three transcription factors directly into the pancreases of diabetic mice turned them into insulin-releasing cells that kept the critters’ blood sugar levels in check. Another study, also in mice, converted heart cells that cause dangerous scarring after a heart attack into healthy heart muscle cells, leading to improved heart function. Scientists have also reprogrammed “supporting” brain cells in mice into functional neurons after brain injury or to treat neurodegenerative diseases.
But these cellular identity swaps all began with relatively normal cells. Tumor cells don’t work the same way—and their abnormalities could torpedo the process.
Tumor Makeover
The new study builds on the team’s previous work reprogramming tumor cells in petri dishes. They aimed to convert these tumor cells into cDC1 cells because of their “manager” role coordinating immune responses.
First, they found three transcription factors that convert other cells into cDC1 cells. Next, they inserted genetic sequences of those factors into a virus stripped of its disease-causing properties. These viral carriers can deliver genes into cultured cells or the body.
As a proof of concept, the team grew melanoma cells in petri dishes, treated some with the gene therapy, and injected the engineered cells into healthy mice. Without the treatment, the melanoma cells rapidly expanded. Cells with the gene therapy, however, couldn’t grow as fast.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


The average survival rate increased from 19 days without treatment to 43 days with it. Adding conventional immunotherapy drugs to the mix cleared all animals of the tumor cells.
The transformed cDC1 cells readily dismantled the tumor’s protective shield. After nine days, more immune cells swarmed the tumor, suggesting its protective barrier had begun to erode.
Classic immunotherapy drugs often exhaust T cells, limiting their expansion and ability to attack. Reprogramming lowered the chances of exhaustion in multiple types of T cells by as much as eight-fold.
Meanwhile, the treatment boosted the number of memory T cells—which, true to their name, retain a ledger of previous targets, including specific cancers. These cells guard the body against cancer resurgence. Once they detect previously defeated tumors, they alert other components of the immune system to strike before the cancer cells can regrow and spread.
Can It Work in the Human Body?
Tumors in mice aren’t exactly the same as those in people. In another test, the team grew little balls of cells from multiple types of immortalized cancer cell lines in petri dishes. Some of these so-called “spheroids” included cells and other factors from a tumor’s protective shield.
Reprogramming the cancer cells into cDC1 cells decreased the size of the cancerous balls, although the efficiency differed between cancer types. Adding common drugs for cancer—which notoriously lower some immune responses—didn’t affect reprogramming and subsequent immune cell activation.
So far, good. But could the therapy work directly inside the body—without having to extract tumor cells and reprogram them in the lab. In a final test, the team injected the treatment into melanoma tumors in mice over the course of two weeks.
Half of those treated remained cancer-free for 100 days, with an abundance of T cells infiltrating the tumor area. The treated mice also readily fought off an experimental model of cancer relapse, holding malignant cells at bay for at least another 60 days—compared to control mice who developed cancers within a month.
There’s a long road before the treatment reaches clinics. But the team is already testing safety profiles, drug metabolism, and scaling up manufacturing processes to get ready for clinical trials.
Turning tumor cells against themselves “offers the advantages of a precision cell therapy, while overcoming the challenges” of genetically engineering immune cells outside the body, as happens in currently approved CAR T therapies, wrote the authors. That said, work that directly engineers CAR T cells inside the body is also on the rise.
Still, results here pave the way for human trials. They lay “the foundation for a new class of immunotherapies based on the unique function” of different types of immune cells, made inside the body using reprogramming, the authors concluded.
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

Your Genes Determine How Long You’ll Live Far More Than Previously Thought
Google DeepMind AI Decodes the Genome a Million ‘Letters’ at a Time
What we’re reading