
Pharmaceutical giant Eli Lily just got FDA approval on a chemical that would enable clinicians to detect a biological marker for Alzheimer’s disease. They can detect the marker now, but currently quantifying it can only be performed during autopsy. Detecting it early, during a person’s lifetime has the potential to not only identify people at risk for Alzheimer’s before they show symptoms, but it could help researchers searching for a cure.
The chemical, called florbetapir or its brand name Amyvid, binds to the protein, beta-amyloid, thought to be a hallmark of Alzheimer’s disease. The drug, which is radioactive, is injected into patients which are then imaged with a Positron Emission Tomography (PET) scan that detects the radioactive signal. A positive scan means there are at least a moderate amount of amyloid plaques, the aggregates of amyloid protein thought to disrupt neuronal function and lead to cognitive decline and dementia associated with Alzheimer’s disease.
Right now Alzheimer’s disease is diagnosed by individual physicians who detect cognitive and behavioral changes in the patient. But by the time behavioral changes are evident, scientists think, the disease is already very advanced. They think that the disease actually begins years before symptoms start to show. If there was a way to detect the disease, such as a PET scan that detects an increase in amyloid protein, doctors could identify high risk patients years before they begin to show symptoms.
Unfortunately, that won’t help the patients very much as there is currently no cure for Alzheimer’s disease. But the real strength of the test rests in its ability to differentiate Alzheimer’s patients who have amyloid with those who do not.
Between 10 and 20 percent of patients diagnosed with Alzheimer’s disease do not show abnormal levels of amyloid at autopsy. The cause of the disease may be different in these patients than in the patients with elevated levels of amyloid, and thus different treatments may be required. Conducting separate studies on these two groups could be a better way to finding a cure.
Researchers also estimate that in some communities a third of patients with mild symptoms but nonetheless have Alzheimer’s disease go undiagnosed. If these patients also show increased levels of amyloid doctors may be quicker to the diagnosis.
Research seeking treatment for Alzheimer’s also stands to benefit from detecting amyloid earlier. Nowadays people have to already show signs of cognitive decline to qualify for clinical trials. The problem is, the damage is already done by the time outward symptoms begin to show. If it turns out that the amount of amyloid increases appreciably before they show cognitive decline, these people at risk to develop Alzheimer’s could be enrolled in clinical trials earlier. And clinical trials aside, just correlating the timing of amyloid increase to the onset of behavioral symptoms could help researchers understand how the two are related.
Amyvid adds to the recent growth of the Alzheimer’s diagnosis toolkit. Tests, shown to be extremely accurate in differentiating Alzheimer’s and normal individuals, are already commercially available to measure amyloid from spinal fluid. A bit less traumatic than a spinal tap, a blood test was developed last year that correctly identified over 80 percent of Alzheimer’s patients based on the levels of nine hormones and proteins including amyloid. And GE Healthcare is currently developing another PET approach that uses a different tracer to bind amyloid, [(18)F] Flutemetamol. Phase III trials for the drug are underway.
Eli Lily said Amyvid will be available this June in “limited quantities.” Side effects from the drug include headache, fatigue, muscle pain, and nausea.
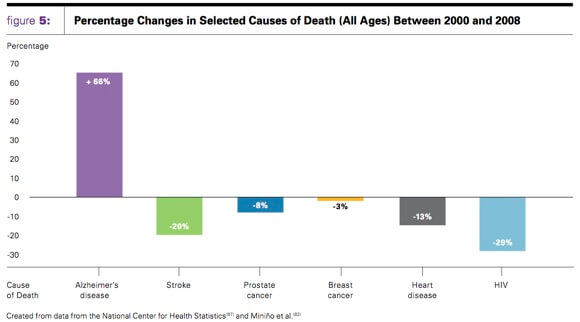
Alzheimer’s disease affects 54 million Americans and is the sixth-leading cause of death in the US. It is a devastating disease, not only for the victims, but their friends and family. Last year unpaid caregivers gave an estimated 17.4 billion hours towards caring for Alzheimer’s patients.
A point that is unfailingly raised in discussions about diagnosing Alzheimer’s disease is the fact that there is no cure. The few drugs that doctors prescribe are only meant to treat symptoms by improving cognitive function or decreasing anxiety. There’s a lot of people who don’t want to know if they’re at high risk for Alzheimer’s disease if they know it can’t be reversed. Fair enough. But whether or not you want to know right now, when there is a cure for Alzheimer’s, it’ll be nice to know we have tools to detect it and detect it early.
[image credits: Alzheimer’s Association and modified from Journal of The American Medical Association]
images 1 and 2: JAMA
image 3: Alzheimer’s
video: Alzheimer’s by the numbers


