Whole Genome Sequencing to Unravel Genetic Basis of Pediatric Cancer
Share
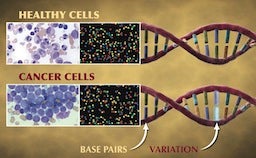
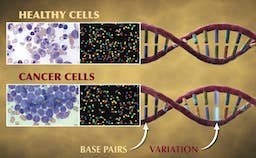
Widely considered to be the world’s leading institution for the study of childhood cancers, St. Jude Children’s Research Hospital recently announced a large-scale genomic study to understand the origins of pediatric cancers. Teaming up with the Genome Center at the Washington University School of Medicine in St. Louis, the 3-year, $65 million Pediatric Cancer Genome Project will sequence the entire genomes of 600 cancer patients. Researchers will sequence both normal, healthy cells and cancer cells from each patient so that they can identify the genetic mutations that lead to the development of cancer. While this is a massive undertaking by today’s standards, these sorts of genomic studies will become more common as the cost of whole genome sequencing continues to drop! Check out the video below where several of the researchers involved with this study describe what we may learn from it.
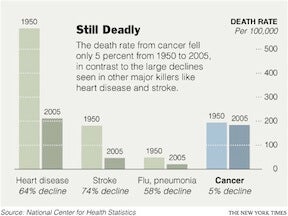
“Cancer is a disease of our DNA.” These are the words of Dr. William E. Evans, the director and chief executive officer of St. Jude, and they accurately describe the disease that many of us are all too familiar with. According to the National Center for Health Statistics, death rates from cancer have fallen a meager 5% since 1950! Compare that to the 64% drop in deaths caused by heart disease during the same period and it’s easy to see why the word “cancer” can seem like a death sentence. According to the National Cancer Institute, the statistics are even worse for children in the US, as cancer is the leading cause of death from disease in children over the age of 1.
Now, looking at statistics like those presented may seem like we have made no advancements in cancer research, but that is hardly the case. Our understanding of the role of carcinogens (i.e. asbestos), lifestyle choices (i.e. smoking), and genetic mutations that increase one’s susceptibility to cancer is becoming more refined every day. One thing that is becoming evident, though, is that cancer is not one disease, but rather a range of diseases with immense complexity, making the likelihood of a “silver bullet” cure quite unlikely. And researchers have long suspected that even cancers that affect the same tissues probably have different causes in children and adults.
The reasoning behind this idea is straightforward: most adult cancers are a result of several mutations that accumulate during one’s lifetime. (Part of the reason why cancer death rates have remained static may be that the increased lifespan provided by advancements in medicine simply allow more time for mutations to occur.) However, young children have simply not lived long enough to accumulate all of these mutations and thus, it’s likely that other mechanisms are involved in causing childhood cancers. The findings of the Pediatric Cancer Genome Project should provide a very clear picture of what these mutations are and how they differ from the mutations found in adult cancers.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


Besides expanding our understanding of what drives cancer development, researchers hope to identify genetic markers that can accurately predict how well a patient will respond to treatment. This should allow development of diagnostic tests that use these markers. Further, this study may also identify drug targets that can help pharmaceutical companies design more effective drugs with fewer side effects. For now, the researchers plan to focus on some of the deadliest pediatric cancers, including leukemia (cancer of the blood), brain cancer, and sarcoma (cancer of connective tissues).
The technology that makes the Pediatric Cancer Genome Project both possible and economically feasible is that of whole genome sequencing. It is undoubtedly one of the most exciting advancements in medicine in the last decade and it has evolved greatly since its origin. Consider the Human Genome Project that was launched in 1990. It took 20 leading research institutions about 13 years and more than $2 billion to decipher the first complete human genome. Today, the time frame is approximately one month (per patient) and the cost is much less, considering 600 patient sequences are expected to cost $65 million. Additionally, commercial companies such as Complete Genomics are working to further decrease cost and turn-around time, so that this technology may be a routine diagnostic tool for diseases beyond cancer. Once that happens, we can truly move into the age of personalized medicine, broadly defined as the use of genomic and proteomic information (among other things) about an individual to identify his disease risk and tailor treatments accordingly. So take heart! It’s only a matter of time before we conquer cancer and other diseases that have claimed so many lives already. And I applaud St. Jude and Washington University for the lead they are taking to get us to that point.
[Image credits: St. Jude Children’s Research Hospital and Washington University School of Medicine in St. Louis, New York Times]
[Video credit: Washington University School of Medicine in St. Louis]
[Sources: Pediatric Cancer Genome Project, National Center for Health Statistics, National Cancer Institute, US Department of Energy]
Related Articles

More Space Junk Is Plummeting to Earth. Earthquake Sensors Can Track It by the Sonic Booms.

Researchers Break Open AI’s Black Box—and Use What They Find Inside to Control It

This Week’s Awesome Tech Stories From Around the Web (Through February 21)
What we’re reading