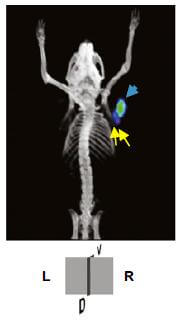
What if you could not only beat cancer, but watch your body do it? Researchers at UCLA’s Jonsson Comprehensive Cancer Center are using gene therapy to teach immune cells how to attack cancer, but that’s now all. By inserting a “reporter gene” that glows under a Positron Emission Tomography (PET) scan, researchers can watch in real-time as the genetically modified immune cells seek out and destroy tumors within the body.
Gene therapy uses a vector (typically an innocuous virus or retrovirus) to insert new genes into the body’s cells. Cancerous cells are usually ignored by the immune system, which does not distinguish them from healthy cells and allows them to divide uncontrollably. The UCLA researcher team, led by Dr. Antoni Ribas, used a crippled virus similar to HIV to upgrade the DNA of lymphocytes; this makes them produce T-cell receptors that allow them to identify and destroy melanoma cells in mice. They also added in a reporter gene that glows “hot” under PET or bioluminescence imaging – this allows them to follow the engineered lymphocytes in real time as they kill cancerous cells.
Ribas’ team performed their research on melanoma grown in mice. After the mice were initially injected with the altered lymphoma cells, PET scans revealed that they had accumulated around and started attacking melanoma tumors after two or three days. The mice were scanned periodically for the next ten days, as scientists confirmed that the lymphocytes were indeed destroying the cancer. We recently reported on a similar study that used reporter genes to follow stem cells injected into the hearts of mice.
Being able to follow particular cells as they travel through the body will be an important step forward for gene therapy research. Currently, if a particular cell line doesn’t work, it’s unclear which step in the immune response failed (i.e. which step to focus on). Reporter genes would allow doctors to determine whether or not injected cells reach their target, even if the cancer isn’t being destroyed. Treatments can then be catered around the specifics of each patient’s response to the gene therapy.
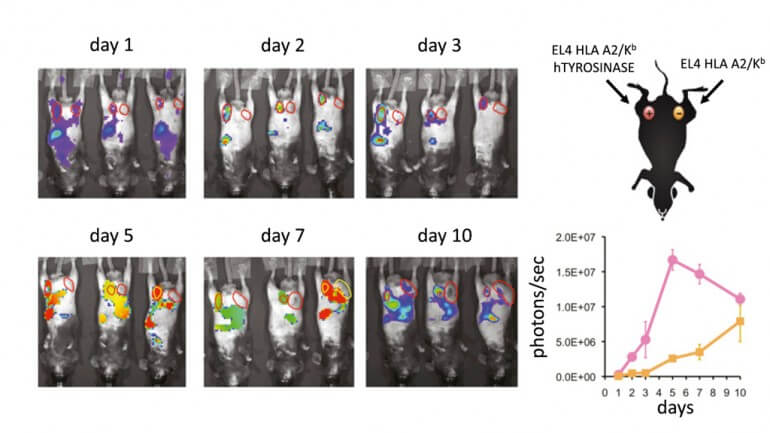
Dr. Ribas and his team are now working on developing a vector that is safe to use in humans, and he estimates that one will be ready within a year. While mouse studies showed the lymphocytes attacking cancer cells within days, human treatments will probably take longer. Engineering the lymphocytes will also take more work; the mice were injected with about one million cells, but the human body will need about one billion to generate a comparable result. The reporter gene labeling is safe and the same technique can be applied directly to humans.
Gene therapy is usually employed to fix or replace problematic genes within the genome. We recently reported on gene therapy trials for bubble boy syndrome which will aim to fix defective immune systems by inserting functional genes that code for lymphocytes. But it’s interesting to note a new trend emerging, beyond just fixing mutations or broken genes. Gene therapy can do more than patch up mistakes in the genome: it can actually be used for upgrades. The body doesn’t normally fight cancer – now it does. Immune cells don’t normally glow so your doctor can watch them – now they do.
What other kinds of genome upgrades might be on the way?


