One particular genetically modified salmon is on the verge of successfully navigating the treacherous regulatory waters of the FDA. The agency just issued a draft environmental assessment of the genetically engineered salmon in which they asserted that the salmon “would not have a significant impact on the US environment.” The agency also concluded the salmon to be “as safe as food from conventional Atlantic salmon.” If all goes as expected, the salmon could be available for consumers by late next year.
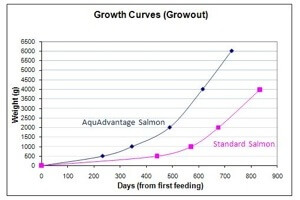
The genetically modified salmon are produced by AquaBounty Technologies, based in Maynard, Massachusetts. Their AquAdvantage salmon are Atlantic salmon that combine a growth hormone gene from Chinook salmon and a control gene from an eel-like creature called the ocean pout that cranks out the growth hormone. The result is that the AquAdvantage salmon grow faster, cutting the time to reach market weight by about half – 18 months compared instead of three years required by normal salmon. Despite the accelerated growth rate, the resultant salmon don’t grow to be any larger than their wild counterparts.
AquaBounty Technologies have sought FDA approval for about 17 years. The FDA had previously determined that the salmon were safe to eat but had yet to rule on whether or not they were safe for the environment. The current draft assessment will be posted on the FDA website for the next 60 days over which time the public is invited to voice concerns over the salmon. Approval is expected to follow the open comment period.
The FDA decision has infuriated some environmental and consumer groups. Andrew Kimbrell, executive director of the advocacy group Center for Food Safety that opposes farm biotechnology, calls the agency’s decision “premature and misguided.” He said in a statement that “the G.E. salmon has no socially redeeming value. It’s bad for the consumer, bad for the salmon industry and bad for the environment.” The group’s statement goes on to say that the FDA’s decision ignores the recommendations of more than 40 members of Congress for more rigorous reviews of the environmental and health effects of the salmon and near 400,000 comments from the public urging the FDA to reject the application.
A major concern among opponents is the risk that the AquAdvantage salmon could escape into the wild and outcompete normal salmon for food or mates. But the multiple layers of protection against this scenario has led the FDA to call the risk “extremely remote.”
First, the salmon would be reared inside inland tanks in Panama that are equipped with multiple barriers to prevent escape. And even if some salmon do manage to pass the barriers the surrounding Panamanian waters would be too warm or salty for the Atlantic fish to survive (eggs are taken from plants on Prince Edward Island, Canada). Lastly, the AquAdvantage salmon will be sterilized and unable to breed, although the sterilization procedure is acknowledged to not be foolproof.
AquaBounty rejects the environmental risk argument and assert that the accelerated growth of their fish and the shortened time to market actually decrease their environmental impact by enabling them to be grown in inland tanks instead of ocean pens that can wear on their ocean surroundings.
It’s important to point out that the FDA only assessed how the salmon might affect surrounding environs in the US, but not in Panama or Canada.
In addition to environmental concerns, opponents are concerned that the FDA’s previous assessment that the AquAdvantage salmon are safe to eat was not thorough enough.
The FDA will take some time, perhaps a few months, to consider comments from consumers before making a final decision. If they maintain their current stance that the salmon are safe an additional approval would then allow the salmon to be sold in the US. Ronald Stotish, AquaBounty’s chief executive, told the New York Times that if the fish are approved next year the genetically modified fish could reach American dinner tables by late 2013. Initial supplies would be limited given that the Panama farm is currently the only one growing the fish. But AquaBounty plans on selling the modified eggs to other fish farms to boost their salmon supply.
The FDA decision is a major victory for genetically modified foods. If the fish do end up on dinner plates across the US – and it certainly appears as though they will – the AquAdvantage salmon could pave the way for genetically modified foods of all kinds. No doubt we are in for a prolonged battle between opposition groups and companies like AquaBounty working to be major players in a potentially extremely profitable market. The battle with play out on our dinner plates as the foods are shown to be safe for us or not, and at the fish farms and crop fields as they are shown to be safe for the environment or not. It’ll be a grand experiment with implications for the future of our food, and with heavily vested interests on all sides.




