Forget sperm meets egg.
Using human stem cells, scientists have created human embryo-like structures inside petri dishes. These lab-grown blobs develop multiple structures that mimic a human embryo after implantation into the uterus—a major milestone for fertility—and last at least 14 days.
A decade ago, manufacturing embryo-like structures, or embryoids, without reproductive cells would have seemed ludicrous. But as scientists increasingly map out the convoluted molecular journey towards human conception, it’s becoming possible to do away with sperm and egg in order to peek into the “black box” of early human development.
It still sounds like a Frankenstein experiment. But the endeavor isn’t macabre scientific curiosity. Very little is known about the first few weeks of human pregnancy, when development most often tends to go awry. Studying models mimicking these early stages—without the controversy of biological samples—could help couples struggling to conceive and shine a light on the mysteries of lost early pregnancies.
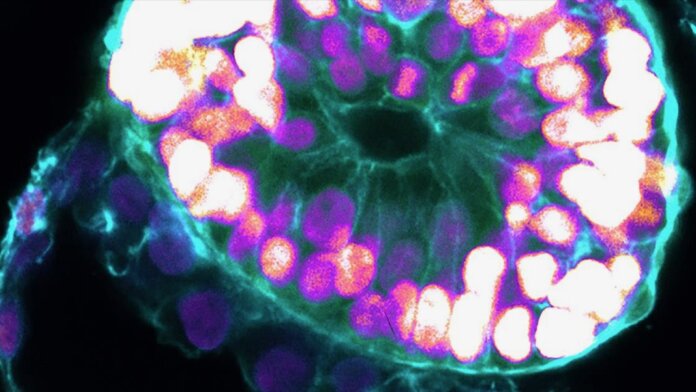
A new study published in Nature from embryoid veteran Dr. Jacob Hanna now pushes the lab-gestation timeline forward. The team turned human embryonic stem cells into embryoids that model early human embryos. Like their biological counterparts, the lab-based blobs developed major “layers” of tissues defining the early stages of human development.
“The drama is in the first month, the remaining eight months of pregnancy are mainly lots of growth,” said Hanna. “But that first month is still largely a black box. Our stem-cell-derived human embryo model offers an ethical and accessible way of peering into this box.”
Recipe for an Embryoid
Two years ago, the same team released a blockbuster result: egg meets sperm isn’t necessary to spark life, at least in mice. Using mouse stem cells, the team discovered a chemical soup that could nudge the cells into embryo-like structures inside a petri dish.
“The embryo is the best organ-making machine and the best 3D bioprinter—we tried to emulate what it does,” said Hanna at the time.
The idea seems relatively simple: all embryonic cells have the potential to become any other cell type. But these cells are also highly social. Depending on their environment—for example, which chemical or hormonal signals they receive—they self-organize into tissues.
Culturing embryoids relies on two advances, both from the Hanna lab.
One places reverted stem cells into a completely naïve state—a tabula rasa that wipes away any identity. We often think of stem cells as a uniform crowd, but they’re actually on a spectrum of development. Each step forward guides the cell’s development towards a specific cell type or organ. However, a naïve stem cell has the potential to grow into any body part.
Completely rebooting to naïve stem cells makes it easier to integrate stem cells into their hosts—regardless of whether it’s in humans or mice.
Another advance is an electronically controlled device that bathes the embryoids in waves of nutrients. Like a pacemaker, the pump simulates how nutrients wash over embryos in the womb, all the while controlling oxygen levels and atmospheric pressure.
In a proof-of-concept study, a small portion of cells from mice formed into embryo-like structures. They developed similarly to their natural counterparts up until roughly half of their normal gestation. By eight days, the embryoids had a beating heart, blood cells in their circulation, a mini-brain with its classical folds, and a digestive tract.
“If you give an embryo the right conditions, its genetic code will function like a pre-set line of dominos, arranged to fall one after the other,” said Hanna in an earlier interview. “Our aim was to recreate those conditions, and now we can watch, in real time, as each domino hits the next one in line.”
Nearly Human
Mice are not men. Hanna is well aware, and the new study bridges the chasm.
The first step? Prime a human stem cell by reverting it into a naïve state.
With this raw material in hand, the team next gave the cells different identities, called lineages. Some of these develop into cells that eventually make up the embryo. Others turn into supporting cells, such as those that make up the placenta or build the yolk sac—a small, rounded multitasker that supports the health of the developing embryo.
In other words, the early developing human embryo is a complex ecosystem. So, it’s no wonder that coaxing naïve stem cells into multiple roles has long eluded embryoid makers. Yet every single lineage becomes indispensable after a major step in early human development, implantation, takes place. When a fertilized embryo attaches to the uterine wall, it sparks a myriad of changes essential for further development. It’s also when embryo loss often occurs.
The new study zooms in on the post-implantation stage, repurposing the team’s previous mouse embryoid protocol to generate self-organizing human embryoids. Surprisingly, it was simpler.
They had to genetically engineer mouse stem cells to push them towards different lineages, the team says. With human cells, they just tweaked the nutrient bath—no additional genes required—to activate genetic programs in stem cells, turning them into all three types of supporting tissues.
As the embryoids matured, the team used a series of molecular and genetic tools to examine their fidelity. Overall, the structures resembled the 3D architecture of naturally developed human embryos between 7 to 14 days after fertilization. Some cells even pumped out human chorionic gonadotropin (hCG), a hormone used for home pregnancy tests. Dabbing the cells’ secretions onto the stick gave the double-line positive result.
Overall, the embryoids showed key developmental landmarks of an early implanted embryo, said the team, without the need for fertilization or interactions with a mother’s womb.
Embryoid Race
Hanna’s team isn’t the only one pushing embryoids forward.
In June this year, two other teams engineered embryoids that mimic human embryos after implantation. The recipes and ingredients are different than Hanna’s. One study, for example, inserted a host of powerful genetic factors that pushed stem cells to become supporting tissues.
Scientists don’t quite agree on which embryoids best resemble their natural counterpart. However, they do agree on one aspect: stem cells, under the right conditions, have an incredible ability to self-organize into increasingly sophisticated embryo-like structures.
For now, the 14-day embryoid is touted as the “most advanced” yet.
Fourteen days is a strict cutoff for research on natural human embryos in many countries, in that they can’t be further cultured in the lab. However, embryoids don’t meet the definition of an embryo and aren’t subjected to the 14-day limitation. In other words, human embryoids could be cultured further along the development timeline. Previous work shows it’s technologically possible in mice, with stem cells developing semi-functional organs.
If you’re getting a bit creeped out—you’re not alone. Embryoids are growing into ever later stages in an arms race to open the black box of early human development. For now, embryoids grown from human embryonic stem cells have to respect current regulations. However, ones made from induced stem cells—often using skin cells reverted into a stem-cell-like state—aren’t subjected to any rules.
To be clear, embryoids don’t have the capacity to fully develop into human beings. However, a recent study in monkeys showed that they can induce pregnancy when transplanted into a womb—though in that case, the embryoid was rapidly and naturally terminated. Debates on if and how to regulate these cellular blobs are ongoing.
For now, Hanna’s team is focused on a revising their recipe to boost efficiency. But as a long-term goal, they hope to push the embryoid even further to see if they can develop rudimentary organs. These experiments “will offer insights into previously inaccessible windows of early human development,” they say.
Image Credit: Weizmann Institute of Science