Brain Implant Sparks Remarkable Recovery in Patients With Severe Brain Injury
Share
At 21 years old, a young woman’s life was turned upside down after suffering a blow to the head and severe brain injury during a devastating traffic accident.
She’s been living with the consequences ever since, struggling to focus long enough to complete simple everyday tasks. Juggling multiple chores was nearly impossible. Her memory would slip. Words would get stuck on the tip of her tongue. Her body seemed to have a mind of its own. Constantly in motion, it was difficult to sit still. Depression and anxiety clouded her mind.
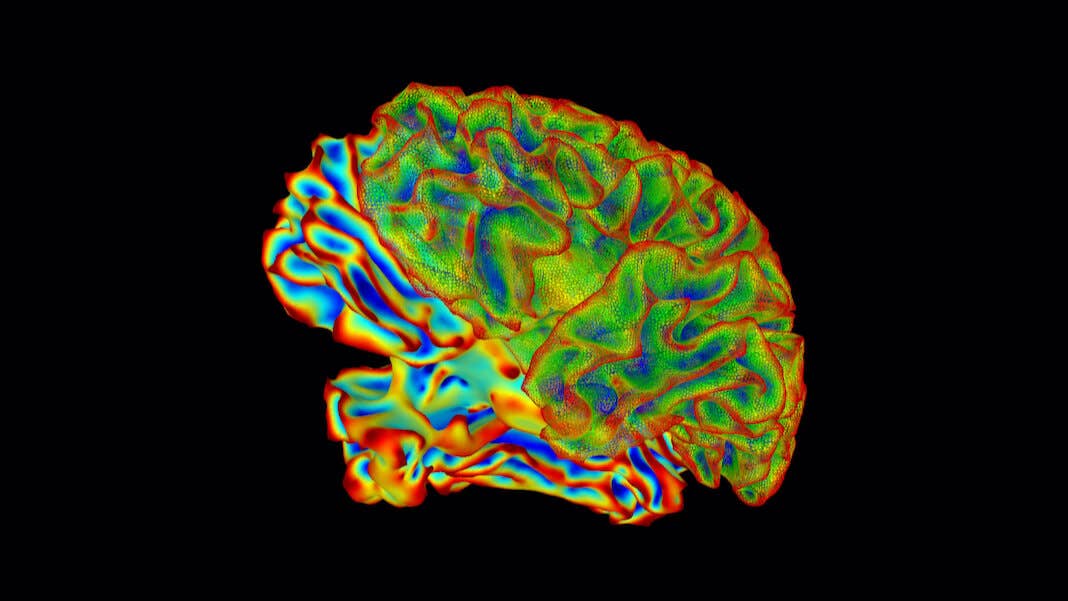
Eighteen years later, she underwent a surgery that again changed her life. After carefully mapping her brain, surgeons implanted electrodes deep into the thalamus. Made of two bulbous structures—one on each hemisphere—the thalamus is the Grand Central Station of the brain, its connections reaching far and wide across multiple regions. A stimulator, implanted near her collar bone, automatically activated the neural implant for 12 hours a day.
The results were striking. In just three months, her scores improved on a standard test measuring myriad cognitive functions. For the first time in decades, she no longer felt overwhelmed throughout her day. She began to love reading and other hobbies.
“I just—I want to think,” she told the researchers. “I am using my mind…I don’t know why, it just makes me laugh, but it’s amazing to me that I enjoy doing these things.”
The woman, known as P1, took part in a small, ambitious trial seeking to reverse cognitive troubles from brain injuries. Led by Dr. Jaimie Henderson at Stanford University, the clinical trial recruited six people to see if electrically stimulating the thalamus restored the participants’ ability to logically reason, make plans, and focus on a given task.
On average, five of the participants’ scores improved by up to 52 percent, far outperforming the team’s modest goals by over five-fold. Because the stimulation is automatic, the volunteers went about their daily lives as the implant worked its therapeutic effects under the hood.
The benefits were noticeable. One participant said he could finally concentrate on TV shows, whereas previously he struggled due to short attention span. Another said he could now track multiple activities and switch attention—like keeping up a conversation while putting groceries away.
While promising, the therapy requires brain surgery, which can be risky. One participant withdrew midway due to infection. But for those who tolerated the therapy, it’s been a life-changer not just for them, but for their families.
“I got my daughter back. It’s a miracle,” said a member of P1’s family.
Tunneling Deep
Deep brain stimulation, the core of the therapy, has a long history.
The idea is simple. The brain relies on multiple circuits working in tandem. These connections can break due to disease or injury, making it impossible for electrical signals to coordinate and form thoughts or decisions.
One solution is to bridge broken brain networks with a neural implant. Thanks to sophisticated implants and AI, we can now tap into the brain and spinal cord’s electrical chatter, decode their intent, and use this “neural code” to drive robotic arms or allow paralyzed people to walk again.
While powerful, these implants often sit on the outer layer of the brain or around nerves in the spinal cord that are relatively easy to access.
Deep brain stimulation presents a challenge because it targets regions buried inside the brain. Invented in the 1980s to treat motor symptoms in Parkinson’s disease, the technology has since been used to battle depression, with just a few zaps easing symptoms in the severely depressed.
The new study built on these results. People with long-term traumatic brain injury often struggle with mood and attention span, making it difficult to balance multiple tasks without headaches and fatigue. They also struggle to sit still.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


These functions are controlled by different areas of the brain. But one critical link is the thalamus, a hub that connects regions supporting attention, mood, and movement. The thalamus is made up of two garlic-shaped bulbs, each nestled in the brain’s hemispheres, that coordinate signals from across the brain. A major sensory relay station, it’s been dubbed “the gateway to consciousness.”
Previous studies in mice pinpointed part of the thalamus as a potential therapeutic hub for traumatic brain injury. Other studies found that stimulating the region was safe in people with minimal consciousness and helped them recover. That’s the region the new study targeted.
Zapping Away
The team narrowed down over 400 volunteers to just six—four men, two women with moderate to severe traumatic brain injury symptoms. Before surgery, they were given multiple tests to gauge their baseline cognitive abilities, mood, and general outlook on life.
Each participant had a commercially available neurostimulator implanted into their thalamus in both brain hemispheres. To catch potential early effects after implantation, they were assigned to three groups based on how soon the implant was turned on post-surgery.
The participants experimented with different zapping patterns for two weeks. Like scrolling through Spotify playlists, each eventually found a pattern optimized to their neural makeup: The stimulation’s timing and intensity allowed them to think clearer and feel better, with minimal side effects. The implant then stimulated their thalamus 12 hours a day for three months.
The results were impressive. Overall, the participants improved between 15 and 52 percent as measured by the same cognitive test used for their baseline. Two patients, including P1, improved so much that they no longer met the diagnosis for lower moderate disability. This boost in mental capacity suggests the participants can tackle work and reconnect with friends and family with minimal struggle, the team wrote in the study.
Another test halted the stimulation in a handful of participants for nearly a month. Neither the researchers nor participants initially knew whose implants were turned off. Within weeks, two patients noticed they felt much worse and withdrew from the test. Of the three people remaining, two improved—and one got worse—with the stimulator on. Further investigation found the implant was erroneously zapping the non-responsive patient’s brain when it should have been turned off.
Although there were minimal side effects, the treatment didn’t disrupt the participant’s lives. The zapping caused some jaw muscle strangeness in a few people. P1, for example, found she slurred her words when on the highest stimulation intensity. Another person had trouble staying still, and some experienced mood changes.
The study is still early, and many questions remain unanswered. For example, does the treatment work regardless of where the brain was injured? The volunteers were only tested for three months after surgery, meaning longer term improvements, if any, remain a mystery. That said, multiple participants signed on to keep their implants and participate in future studies.
Even with these caveats, participants and their loved ones were thankful. “It’s so profound to us,” P1’s family member said. “I never would’ve believed it. It’s beyond my hopes, beyond anticipation. Somebody turned the lights back on.”
Image Credit: National Institute of Mental Health, National Institutes of Health
Dr. Shelly Xuelai Fan is a neuroscientist-turned-science-writer. She's fascinated with research about the brain, AI, longevity, biotech, and especially their intersection. As a digital nomad, she enjoys exploring new cultures, local foods, and the great outdoors.
Related Articles
New Gene Drive Stops the Spread of Malaria—Without Killing Any Mosquitoes
New Immune Treatment May Suppress HIV—No Daily Pills Required

Scientists Just Developed a Lasting Vaccine to Prevent Deadly Allergic Reactions
What we’re reading