The last woolly mammoth roamed the vast arctic tundra 4,000 years ago. Their genes still live on in a majestic animal today—the Asian elephant.
With 99.6 percent similarity in their genetic makeup, Asian elephants are the perfect starting point for a bold plan to bring the mammoth—or something close to it—back from extinction. The project, launched by biotechnology company Colossal in 2021, raised eyebrows for its moonshot goal.
The overall playbook sounds straightforward.
The first step is to sequence and compare the genomes of mammoth and elephant. Next, scientists will identify the genes behind the physical traits—long hair, fatty deposits—that allowed mammoths to thrive in freezing temperatures and then insert them into elephant cells using gene editing. Finally, the team will transfer the nucleus—which houses DNA—from the edited cells into an elephant egg and implant the embryo into a surrogate.
The problem? Asian elephants are endangered, and their cells—especially eggs—are hard to come by.
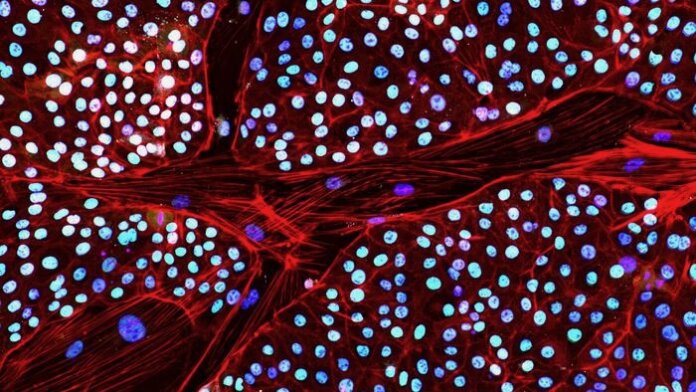
Last week, the company reported a major workaround. For the first time, they transformed elephant skin cells into stem cells, each with the potential to become any cell or tissue in the body.
The advance makes it easier to validate gene editing results in the lab before committing to a potential pregnancy—which lasts up to 22 months for elephants. Scientists could, for example, coax the engineered elephant stem cells to become hair cells and test for gene edits that give the mammoth its iconic thick, warm coat.
These induced pluripotent stem cells, or iPSCs, have been especially hard to make from elephant cells. The animals “are a very special species and we have only just begun to scratch the surface of their fundamental biology,” said Dr. Eriona Hysolli, who heads up biosciences at Colossal, in a press release.
Because the approach only needs a skin sample from an Asian elephant, it goes a long way to protecting the endangered species. The technology could also support conservation for living elephants by providing breeding programs with artificial eggs made from skin cells.
“Elephants might get the ‘hardest to reprogram’ prize,” said Dr. George Church, a Harvard geneticist and Colossal cofounder, “but learning how to do it anyway will help many other studies, especially on endangered species.”
Turn Back the Clock
Nearly two decades ago, Japanese biologist Dr. Shinya Yamanaka revolutionized biology by restoring mature cells to a stem cell-like state.
First demonstrated in mice, the Nobel Prize-winning technique requires only four proteins, together called the Yamanaka factors. The reprogrammed cells, often derived from skin cells, can develop into a range of tissues with further chemical guidance.
Induced pluripotent stem cells (iPSCs), as they’re called, have transformed biology. They’re critical to the process of building brain organoids—miniature balls of neurons that spark with activity—and can be coaxed into egg cells or models of early human embryos.
The technology is well-established for mice and humans. Not so for elephants. “In the past, a multitude of attempts to generate elephant iPSCs have not been fruitful,” said Hysolli.
Most elephant cells died when treated with the standard recipe. Others turned into “zombie” senescent cells—living but unable to perform their usual biological functions—or had little change from their original identity.
Further sleuthing found the culprit: A protein called TP53. Known for its ability to fight off cancer, the protein is often dubbed the genetic gatekeeper. When the gene for TP53 is turned on, the protein urges pre-cancerous cells to self-destruct without harming their neighbors.
Unfortunately, TP53 also hinders iPSC reprogramming. Some of the Yamanaka factors mimic the first stages of cancer growth which could cause edited cells to self-destruct. Elephants have a hefty 29 copies of the “protector” gene. Together, they could easily squash cells with mutated DNA, including those that have had their genes edited.
“We knew p53 was going to be a big deal,” Church told the New York Times.
To get around the gatekeeper, the team devised a chemical cocktail to inhibit TP53 production. With a subsequent dose of the reprogramming factors, they were able to make the first elephant iPSCs out of skin cells.
A series of tests showed the transformed cells looked and behaved as expected. They had genes and protein markers often seen in stem cells. When allowed to further develop into a cluster of cells, they formed a three-layered structure critical for early embryo development.
“We’ve been really waiting for these things desperately,” Church told Nature. The team published their results, which have not yet been peer-reviewed, on the preprint server bioRxiv.
Long Road Ahead
The company’s current playbook for bringing back the mammoth relies on cloning technologies, not iPSCs.
But the cells are valuable as proxies for elephant egg cells or even embryos, allowing the scientists to continue their work without harming endangered animals.
They may, for example, transform the new stem cells into egg or sperm cells—a feat so far only achieved in mice—for further genetic editing. Another idea is to directly transform them into embryo-like structures equipped with mammoth genes.
The company is also looking into developing artificial wombs to help nurture any edited embryos and potentially bring them to term. In 2017, an artificial womb gave birth to a healthy lamb, and artificial wombs are now moving towards human trials. These systems would lessen the need for elephant surrogates and avoid putting their natural reproductive cycles at risk.
As the study is a preprint, its results haven’t yet been vetted by other experts in the field. Many questions remain. For example, do the reprogrammed cells maintain their stem cell status? Can they be transformed into multiple tissue types on demand?
Reviving the mammoth is Colossal’s ultimate goal. But Dr. Vincent Lynch at the University of Buffalo, who has long tried to make iPSCs from elephants, thinks the results could have a broader reach.
Elephants are remarkably resistant to cancer. No one knows why. Because the study’s iPSCs are stripped of TP53, a cancer-protective gene, they could help scientists identify the genetic code that allows elephants to fight tumors and potentially inspire new treatments for us as well.
Next, the team hopes to recreate mammoth traits—such as long hair and fatty deposits—in cell and animal models made from gene-edited elephant cells. If all goes well, they’ll employ a technique like the one used to clone Dolly the sheep to birth the first calves.
Whether these animals can be called mammoths is still up for debate. Their genome won’t exactly match the extinct species. Further, animal biology and behavior strongly depend on interactions with the environment. Our climate has changed dramatically since mammoths went extinct 4,000 years ago. The Arctic tundra—their old home—is rapidly melting. Can the resurrected animals adjust to an environment they weren’t adapted to roam?
Animals also learn from each other. Without a living mammoth to show a calf how to be a mammoth in its natural habitat, it may adopt a completely different set of behaviors.
Colossal has a general plan to tackle these difficult questions. In the meantime, the work will help the project make headway without putting elephants at risk, according to Church.
“This is a momentous step,” said Ben Lamm, cofounder and CEO of Colossal. “Each step brings us closer to our long-term goals of bringing back this iconic species.”
Image Credit: Colossal Biosciences