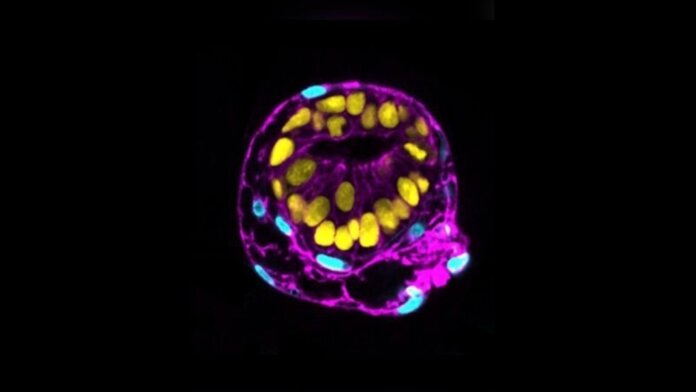
Transforming human stem cells into embryo-like structures was previously unthinkable.
Yet seemingly overnight, multiple teams published initial results that reach towards this goal. Each team has a unique recipe for generating lab-grown embryoids, blobs of cells that mimic aspects of the earliest stages of human life.
Although often dubbed “synthetic embryos,” they are anything but. The 3D cellular clumps are highly intricate, with some beginning to diverge into lineages of cells needed to support growth of the embryo into a fetus. Yet the models are far from their sperm-meets-egg real-life counterparts.
Each embryo-like structure—scientists haven’t yet settled on calling them “embryoids” or “stembryos”—partially replicates genetic, molecular, and cellular aspects of a human embryo up to roughly two weeks after implantation. But the structures all disintegrate after a few weeks. They can’t be transferred into an actual womb, and certainly can’t develop into a viable fetus.
The controversial field isn’t aiming to engineer human embryos from scratch. Rather, it hopes to shine a light on the black box of the first weeks after human conception, and potentially offer a lifeline to couples with infertility or to combat miscarriage.
“This is the stage…where most pregnancies fail for unknown reasons,” said Dr. Berna Sozen at Yale University, who led one effort published in Nature. “Our model platform captures a specific snapshot of human development about which we know perhaps the least.”
The Reproductive Black Box
The first weeks after conception are the ultimate enigma in human development.
We know the basics: a single fertilized egg expands to roughly 200 cells, forming a hollow blob that attaches to the uterine wall, a process called implantation.
The next few days lay the foundation for development. The embryo rapidly grows into three diverse layers, each with its own distinct cell lineage. One forms the “core” of the body, the epiblast, which contains cells that make up the embryo. The second is the hypoblast, which helps orient the main axes of the embryo—put simply, sketching the overall design of the human body—and further forms the supportive yolk sac. Finally, there’s the trophoblast, which gives rise to the placenta to provide nutrients for the growing fetus.
These are only broad brushstrokes. Due to ethical, technological, and regulatory restrictions, we know little of the intricacies behind these processes, including when and how they go wrong. It’s why scientists have been striving for a more ethically acceptable replacement: embryo-like models made from human stem cells. Two teams came close in 2021, reconstructing critical events similar to the first four days after fertilization. The ultimate goal is to mimic all three lineages—the holy grail for reproductive research—and potentially push the developmental time frame further along.
The question is, how?
Cracking the Black Box
Sozen’s team started with human pluripotent stem cells. These multi-taskers happily renew themselves and can develop into almost every single cell type in the body.
When bathed in a chemical soup, the cells spontaneously organized into 3D structures within 48 hours. The recipe was the secret touch: normally, the cells develop into disorganized aggregates that hardly resemble an early human embryo. Here, however, the cells expressed protein markers and formed structures that resembled early-stage epiblasts and hypoblasts after implantation, all the while adopting an embryo’s typical spherical shape.
To further test the cells’ function, the team injected the lab-grown hypoblast cells—ones that usually help orient the body’s blueprint—into early mouse embryos. Less than a third took hold. However, those that did integrated into their new hosts, and remained after the chimeric embryos were transplanted into a surrogate (no mouse babies were born).
Digging deeper, Sozen’s team examined gene expression in single cells from the embryo models. The results further verified that their recipe cooked up two cell lineages, with their “extra-embryoids” showing genetic patterns strikingly similar to their human embryo counterparts, but lacking signs of forming the placenta. The blobs also weren’t able to capture the epigenetic landscape—the control over gene expression without altering its sequence—that’s highly prominent during implantation.
Still, the team is happy with their results. The platform, they explain, uses only one cell type and is scalable and versatile. It’ll help “dissect the mechanisms underpinning early fate decisions occurring at inaccessible stages of our species’ development,” and potentially the origins of developmental disorders, said study author Monique Pedroza.
The work “is a remarkable study that has been carried out with great care,” said Dr. Roger Sturmey at the University of Manchester, who was not involved in the work. Sturmey is also the chair for the G-SCBEM (Governance of Stem Cell-Based Embryo Models) Guidelines Working Group, which aims to establish ethical and regulatory guidelines for the increasingly heated field. “This work describes an extremely important model to support our pursuit of understanding the cellular and molecular events that occur around the time that the early embryo implants into the uterus in early pregnancy,” he said.
A Multiverse of Methods
Meanwhile, in a sister paper published in Nature, embryoid veteran Dr. Magdelena Zernicka-Goetz at the University of Cambridge—a previous advisor to Sozen—took a different method. Rather than changing the external bath recipe, they directly tapped into the genetic program guiding embryoid development.
Zernicka-Goetz is no stranger to engineering embryo-like structures from stem cells. Back in 2022, her lab made headlines for building the beginnings of an embryoid using mouse embryonic stem cells (as did another leading expert, Dr. Jacob Hanna at the Weizmann Institute in Israel). The resulting structure contained all three potential cell lineages and roughly resembled their natural counterparts at 8.5 days old.
The new study adopts a similar method. The key is transcription factors, a group of proteins that help control how genes turn on or off. The goal, explained the team, is to over-express certain factors and push cells into “genetic programs” that help form different cell lineages during development.
The strategy worked—but only partially. By genetically adding the transcription factors, the model skipped roughly a week of “normal” development to form a ball-like structure similar to a post-implantation embryo. The embryoids self-organized into a primitive body axis—the head-to-toe patterning critical to this developmental stage. Further deep dives into the molecular mechanisms identified several biomolecules that help orchestrate this patterning.
Although the strategy did not form the trophoblast—the elusive golden goose lineage that eventually forms the placenta—the results “highlight the value” of using embryoids to study how embryonic and supporting tissues interact at an early stage, the authors said.
Slow and Steady?
In addition to the two published papers, other giants in the field have put forth their own gambit towards an accurate human embryo imitation on a preprint server.
Hanna, who led an effort to build an early mouse embryo, describes a method to achieve the same for human cells—forming embryoids that mimic a 14-day-old natural human embryo, including the elusive trophoblast. Meanwhile, Dr. Mo Ebrahimkhani at the University of Pittsburgh describes a reprogramming method using human induced pluripotent stem cells (iPSCs) and rewired with a synthetic gene circuit to develop both the embryo and its surrounding tissues. According to Stat, the studies are currently under peer review.
Without doubt, the race to build embryoids that resemble the real thing is becoming increasingly heated. The question is: where’s the red line?
In Sturmey’s (the chair of G-SCBEM’s Guidelines Working Group) opinion, we need answers soon. The group, led by scientists and legal and bioethics experts, is leading the charge to establish an ethical path forward for embryoid research. Although mainly established for UK research, the ensuing guidelines pave the path for an international agreement.
G-SCBEM aims to publish their first unified proposal in November and welcomes guidance from others in the field. It may be a hard sell; competition in the field is fierce. But establishing rules for such a complex and ethically ambiguous field, especially if public opinion can be incorporated, will help in the long run—and hopefully avoid another CRISPR baby scandal.
The current race “further illustrates the necessity for a coherent set of guidelines supporting work of this nature,” said Sturmey.
Image Credit: Monique Pedroza, Ipek Gassaloglu, Berna Sozen/Yale University