A Nasty Virus That Infects Bacteria Could Be Key to Improved Gene Therapies
Share
Gene therapies could revolutionize medicine, but getting them into peoples' bodies is harder than it might seem. A new method that re-purposes viruses that infect bacteria could provide a solution.
Finding ways to modify the DNA in the cells of living people could help treat or prevent a host of genetic diseases. It could also help re-purpose their cells to hunt down cancer or produce therapeutic molecules that could treat non-genetic conditions. But while our gene-editing tools are becoming increasingly sophisticated, getting them into peoples’ bodies is complicated.
A few gene therapies exist today, and they mostly use modified viruses, which excel at sneaking their DNA into their hosts' cells. This makes these so-called viral vectors perfect cargo carriers for the tools and genetic material required to edit genes inside patients cells. But the adeno-associated viruses (AAVs) and lentiviruses that are most commonly used have a pretty small carrying capacity, which severely limits the scope of problems they can tackle.
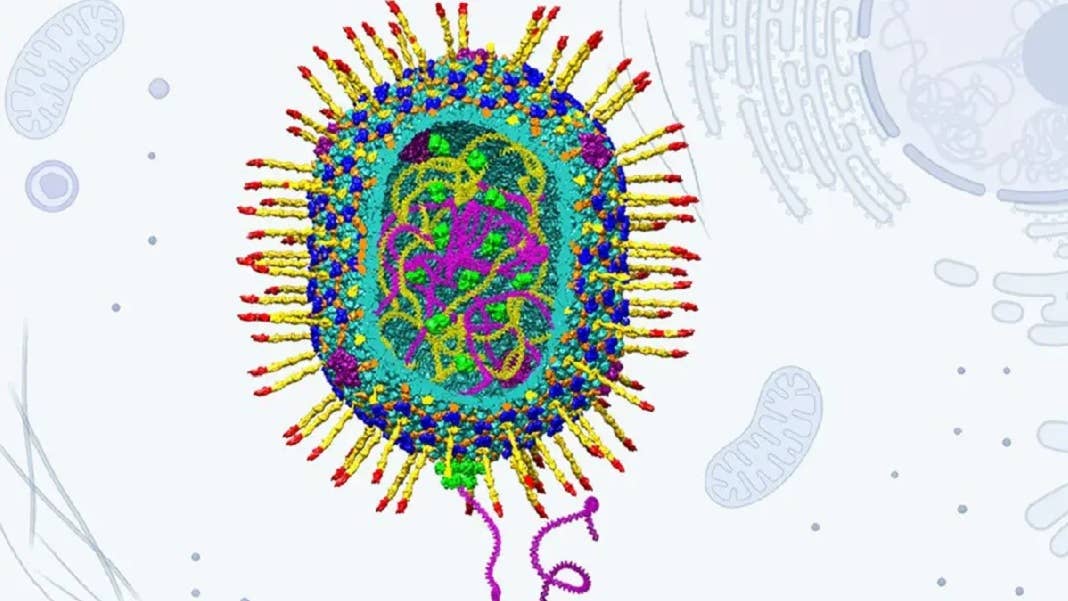
New research from the Catholic University of America has shown that a type of bacteriophage—viruses that infect bacteria—with a much bigger cargo hold can be repurposed to deliver gene therapies. It’s also cheap to make, stable, and easy to program to carry out more complex missions.
“The actual therapy is years down the road, but this research provides a model for developing life saving treatments and cures,” Venigalla Rao, who led the research, said in a press release. “What we are researching is like a molecular surgery that can safely and precisely correct a defect and generate therapeutic outcomes and some day cures.”
In the hunt for a more capable delivery vehicle, the researchers turned to a phage called T4, which belongs to the Straboviridae family and infects E. coli bacteria. It has a host of promising characteristics, including a much larger capsid (the main compartment where genetic material is stored), an infection efficiency of nearly 100 percent, and the ability to replicate in just 20 to 30 minutes.
What’s more, researchers have already worked out the atomic structures of the phage’s main components, making the re-engineering process much simpler. This made it possible for the group to set up what it called an “assembly-line approach” in which cargo molecules like DNA, proteins, and RNA were sequentially added to the empty capsid shells and also stuck on their outside as well. The resulting viral vector is then coated in an envelope of lipid molecules, which make it easier to infiltrate human cells.
In a paper in Nature Communications, the researchers showed that their engineered phage could hold stretches of DNA up to 171,000 base pairs long, which is roughly 20 times more than viruses used in current gene therapies can hold. To demonstrate the potential, they used this carrying capacity to deliver the entire gene for the protein dystrophin into human cells. Mutations in this gene are responsible for the genetic disorder Duchenne muscular dystrophy.
Be Part of the Future
Sign up to receive top stories about groundbreaking technologies and visionary thinkers from SingularityHub.


In a series of experiments, the researchers showed that the viral vector could be used to do genome editing, gene recombination, gene replacement, gene expression, and gene silencing. They also showed that it could carry complex cargoes made up of multiple stretches of DNA aimed at different genes, alongside various proteins and RNA sequences. The researchers say this could ultimately open the door to treating complex diseases that involve multiple genes like many cancers, neurodegenerative disorders, and cardiovascular diseases.
While these early results are certainly promising, Jeffrey Chamberlain at the University of Washington in Seattle told New Scientist that the team has yet to show the viruses can actually deliver genes into the body, rather than simply to human cells in a petri dish. And Rao concedes that there’s still plenty of work to do to make the jump from the lab bench to the clinic.
But the ability to custom engineer viral vectors for a wide range of applications using their assembly line is highly promising. And unlike existing viral vectors, which have to be reared in human cell cultures at considerable cost, the team’s new engineered phage can be grown far more simply in bacteria.
It’s likely to take many more years of research to bring these ideas to fruition, but if successful, this could greatly expand the scope of future gene therapies.
Image Credit: Venigalla B. Rao; Victor Padilla-Sanchez, Andrei Fokine, and Jingen Zhu. Structural model of bacteriophage T4 artificial viral vector.
Related Articles

Souped-Up CRISPR Gene Editor Replicates and Spreads Like a Virus

This Brain Pattern Could Signal the Moment Consciousness Slips Away
Vast ‘Blobs’ of Rock Have Stabilized Earth’s Magnetic Field for Hundreds of Millions of Years
What we’re reading
